| |
Systemic Therapy for Metastatic Disease |

Breast Cancer Update 2006 (7)
DR TRIPATHY: The main endpoint of
ECOG-E2100, progression-free survival,
was significantly prolonged with
the combination of bevacizumab and
paclitaxel compared to paclitaxel alone.
The hazard rates indicate a more robust
improvement than we’ve seen with single
chemotherapy compared to chemotherapy
doublets.
Much attention has been given
to the survival difference, which was
statistically significant when initially presented
at ASCO but was not significant at
the next two presentations at ECCO and
San Antonio. It’s important to remember
that the number of events was nowhere
near what was projected for that analysis.
So although survival is an important endpoint,
I don’t believe the trial had enough
power to demonstrate whether a survival
advantage exists. In the end, data on overall
survival will be important in deciding
whether to use bevacizumab. But right
now, you have to go with the data on progression-free survival.
I have tried to practice the way the
trial was designed, using bevacizumab
for patients only as first-line therapy.
I use it with paclitaxel, and I tend to
reserve it either for patients who are
symptomatic or for those who may not
be symptomatic but whose disease trajectory
is such that I would predict they
might become symptomatic soon. It’s a
judgment call.
In terms of whether or not we might
want to generalize this and combine it
with other chemotherapeutic drugs, I
believe that’s a reasonable consideration.
For patients who have already received a
taxane in the adjuvant setting, should we
use a drug like capecitabine? I believe it
would be reasonable.
Breast Cancer Update — Think Tank
Issue 2, 2006
DR RUGO: In addition to evaluating
the combination of capecitabine/bevacizumab,
another research strategy is to
combine endocrine therapy with bevacizumab.
Some interesting data indicate
that estrogen may directly modulate
angiogenesis through effects on
endothelial cells in both physiologic
and pathologic conditions. We also have
data indicating that antiestrogen therapy
blocks VEGF expression and estrogen-induced
angiogenesis may be blocked by
antiestrogen therapy.
Rakesh Jain’s group in Boston has
observed an androgen-dependent tumor
model and shown that castration, interestingly,
leads to initial vascular regression,
and then a second wave of angiogenesis
occurs with vascular regrowth in
this murine tumor model.
So a hypothesis was generated that
anti-VEGF therapy may overcome this
resistance of the second wave of angiogenesis
seen with endocrine therapy in
animal models and could improve the
efficacy of standard hormone therapy
in hormone receptor-positive metastatic
breast cancer.
In the study presented by Dr Traina
at ASCO this year, 43 patients received
bevacizumab at 15 mg/kg every three
weeks and letrozole at 2.5 mg per day.
The combination appeared to be well
tolerated. The drug-related toxicities
were expected and only seen in a small
number of patients. The efficacy analysis, which wasn’t the primary goal, was
confounded by the long duration of
prestudy aromatase inhibitor therapy in
most patients, although it did appear
that a number might have benefited from
the therapy.
We have planned a Phase III study
within CALGB and the Intergroup in
patients with hormone receptor-positive
disease. The patients will be randomly
assigned to endocrine therapy with
placebo or bevacizumab (administered
every three weeks) as first-line therapy.
Breast Cancer Update — Think Tank
Issue 2, 2006
DR WILLIAM J GRADISHAR: Right now,
we have positive data from ECOG-E2100.
By that, I’m emphasizing the
fact that it’s used in the first-line setting.
I have no reason to believe bevacizumab
in conjunction with other agents, as
first-line therapy, wouldn’t have a similar
benefit. I don’t believe we will see people
restricting themselves to the use of bevacizumab
with paclitaxel alone, but we
don’t have a lot of Phase II data for
combining bevacizumab with a variety of
different agents.
That said, the experience with trastuzumab
is similar — we had preclinical
models that guided us and then
the Phase II trials followed. They all
were consistent in that they demonstrated
an incremental improvement
when you combined the given agent with
trastuzumab. I believe that when bevacizumab
is combined with other chemotherapy
agents, we will see the same
improvement in outcome that we’ve seen
in ECOG-E2100.
Breast Cancer Update 2005 (9)
DR BURSTEIN: We have data for bevacizumab
in combination with paclitaxel.
We certainly use a lot of weekly paclitaxel
as first-line treatment for advanced breast
cancer, so for patients who are already
receiving paclitaxel, I believe this is clearly
the regimen of choice.
The challenge is how to treat patients
in the second- and third-line settings.
At present, there really are only minimal
data to indicate that bevacizumab
is beneficial for such patients. Another
challenge is what to do for those women
who received anthracyclines and taxanes
in the adjuvant setting.
Do you rechallenge them with
paclitaxel and bevacizumab? There are
two halves to that question. The first
is, does bevacizumab actually help these
women? We haven’t seen the data as yet
broken out as a function of prior taxane
therapy. The second half of the question
is should you give the taxane again?
Again, we don’t have good answers. If it’s
been more than a year, it’s probably reasonable
to give the paclitaxel again.
Occasionally, we recommend our
vinorelbine regimen, because of our
Phase II experience with vinorelbine plus
bevacizumab.
Some people administer capecitabine
plus bevacizumab, because, of course,
there are safety data for that. On the
other hand, those data don’t really suggest
that particular combination does
all that much compared to capecitabine
alone. We’re all looking forward to more
studies, more Phase II trials, to really try
and understand how best to utilize this
drug for metastatic disease.
Breast Cancer Update — Think Tank
Issue 2, 2006
DR RUGO: ECOG-E2100 is a significant
advance. Having participated in the
initial capecitabine/bevacizumab trial and also having used bevacizumab in a
variety of clinical research settings, we’ve
been convinced for a long time that it has
clinical benefit.
ECOG-E2100 produced two important
implications. One is that we can,
potentially, help patients in the metastatic
setting with first-line therapy in combination
with a taxane. The second is
that it allowed us to move bevacizumab
into trials in the early adjuvant setting,
as well as into the neoadjuvant setting,
which potentially allows us to identify
the patient population most likely to
benefit from bevacizumab.
Breast Cancer Update 2006 (7)
DR CARLSON: It’s reasonable to offer
patients with triple-negative disease
chemotherapy and bevacizumab as first-line
therapy. The best evidence we have
is with paclitaxel/bevacizumab. Kathy
Miller’s ECOG study that evaluated
capecitabine with or without bevacizumab
showed a slightly higher response rate
using the combination but no advantage
in terms of relapse-free survival and overall
survival.
We may be seeing specific drug effects
and different drug interactions between
bevacizumab and chemotherapy. It may
be a result of different patient populations.
The patients in the capecitabine
study were treated in the second-line
setting, not the first-line setting, as with
paclitaxel and bevacizumab.
Breast Cancer Update 2006 (8)
DR BURSTEIN: For patients with triple-negative
tumors we don’t have a target,
so the work focused on optimizing
chemotherapy. Some trials are evaluating
adding products like capecitabine,
and some are evaluating platinum-based
chemotherapy. Additionally, there is
interest in other biological approaches,
and probably the one that is furthest
along has been to add bevacizumab to
the treatment of these patients. ECOG-E2100
indicated that the patients with
ER-negative, HER2-negative disease did
handsomely with paclitaxel and bevacizumab.
So that is a reasonable patient population in which to try optimizing
chemotherapy and other biological
approaches.
For a woman with visceral, triple-negative
metastatic disease that is extensive
and symptomatic, obviously, we will
administer chemotherapy. Most frequently,
I use paclitaxel with bevacizumab
for patients like that.
I find the data from ECOG-E2100
compelling — we can do better than
using chemotherapy alone by adding
bevacizumab treatment. I like the idea of
using a relatively exciting biological therapy.
The other point is that few women
who walk in the door are chemotherapy
naïve at that point.
Breast Cancer Update 2006 (8)
DR ROBERT B LIVINGSTON: We do
not have hard evidence that one chemotherapy
regimen is better than another
chemotherapy regimen for the patient
with serious, moderately symptomatic
triple-negative metastatic disease. I
believe most of us would be inclined to
use anthracycline-based therapy if the
patient hadn’t received it previously or
if it had been more than a year since
completion of her adjuvant treatment.
Many of us would be inclined to
use a combination rather than a single
agent, and I’m one of those because these
patients have particularly aggressive disease
and tend to experience short times
to progression. The delay in time to progression
that one sees with combinations
may be important for patients with this
type of disease.
At both my earlier institutional affiliations
in Seattle and in the Southwest
Oncology Group, we have been exploring
antitubulin combinations, investigating
combinations of vinorelbine and
a taxane, either docetaxel or paclitaxel.
Most recently, I’ve been involved in a
trial with nab paclitaxel and vinorelbine.
Those combinations are active. What I
can honestly tell you is they’re probably
not more active than somebody else’s
choice of docetaxel and capecitabine or
gemcitabine-based therapy.
The only patient right now, outside of
a study, for whom I would probably urge the use of bevacizumab is this type of
individual, because we do have evidence
that the taxanes are as active, if not more
active, than any other drugs. We do have
evidence that weekly paclitaxel, which is
the best way to administer the drug, is
potentiated by the use of bevacizumab.
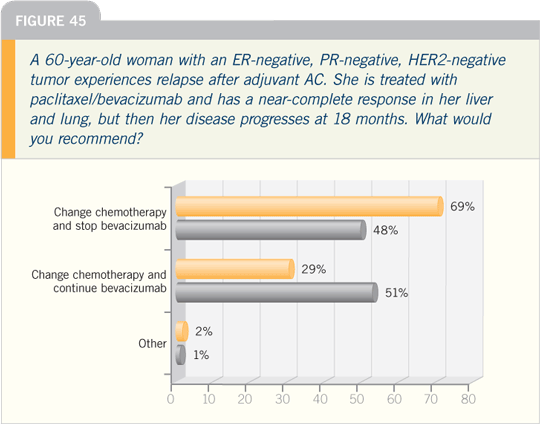
And we do have, in the triple negatives,
a group of patients for whom, right
now, no targeted therapy is available,
except bevacizumab, that we can justify
on the basis of a randomized trial. So if
I were seeing such a patient in the clinic
today, I would talk to her about a taxane-based
treatment program, in all likelihood,
and I would recommend that she
also receive bevacizumab.
Interview, September 2006
DR GEORGE W SLEDGE JR: In ECOG-E2100
the progression-free survivals are
now approximately a year for the combination
of bevacizumab and paclitaxel. If
we saw progression-free survivals in the
same ballpark in the XCaliBr trial evaluating
bevacizumab and capecitabine as
first-line therapy, I believe we’d all find
that very exciting, and it would certainly
suggest that we might be able to combine
bevacizumab successfully with other
chemotherapeutic agents in a more up-front
population.
It becomes important in an era when
patients are receiving more and more
of their therapy in the adjuvant setting,
or more intensive chemotherapy in
the adjuvant setting, so that drugs like
capecitabine might be a preferential first
choice for many patients in the front-line
metastatic setting.
Breast Cancer Update — Think Tank
Issue 2, 2006
DR HUDIS: I believe any patient with
Stage IV breast cancer who is healthy
enough to receive bevacizumab deserves
a shot at capecitabine. I don’t buy the
argument that it only works in the first-line
setting and that it only works with
paclitaxel.
The reasons I say that are, first, the
drug has been extensively used with a
variety of other chemotherapy agents. I
don’t have to see safety data for a drug
specifically in patients with breast cancer
to call it safe. We have a lot of safety data
for the 5-FU/bevacizumab combination.
Second, I thought the capecitabine/bevacizumab trial by Dr Miller was a
positive signal. It showed a doubling of
the response rate, but it did not achieve
its primary endpoint of progression-free
survival. The third reason is that you
can see two patients in a clinic who are
both ready to receive first-line therapy
but have extraordinarily different prior
chemotherapy experiences and exposure.
For all these reasons, I offer bevacizumab,
essentially, to all eligible patients with
a line of therapy at some point in time.
Breast Cancer Update — Think Tank
Issue 2, 2006
DR RUGO: We need the data from the
ongoing RIBBON 1 and RIBBON 2
trials. The trials randomly assign patients
either in the first- or second-line setting
to receive chemotherapy with placebo
or bevacizumab, and then they allow a
crossover. The potential exists to obtain
a lot of information. We have a menu of
chemotherapy agents to choose from in
those settings.
Breast Cancer Update 2006 (7)
DR CARLSON: Capecitabine is often the
chemotherapeutic agent that I use as
first-line therapy. Capecitabine has efficacy
that is in the ballpark of any single
agent, and I tend to treat metastatic
breast cancer that’s not in visceral crisis
with single-agent therapy.
The toxicity profile of capecitabine
is favorable, and the women appreciate
being able to take an oral medication, not
having to go to the infusion center and
not having to come back as frequently. It’s
an agent that, at doses that are typically
used, is associated with a predictable toxicity
experience. I use 1,000 mg/m2 twice
daily — two weeks out of three weeks.
It’s very important that capecitabine
does not cause alopecia. If you’re going
to use sequential single agents, it’s always
nice to start with an agent that doesn’t
cause alopecia. If the woman already has
established alopecia, you don’t gain from
the nonalopecia properties of the new
therapy. That’s often an important component
of treatment of metastatic disease.
The other reason I often will lead
with capecitabine is that many of these
women, because it’s the first-line therapy,
have recently been diagnosed with their
metastasis. They will go through all the
turmoil and psychic trauma of the new
diagnosis, and in that context, often it
is easier to start with an agent that has acceptable toxicity, so they can become
used to the chronic nature of the disease
and the need for ongoing chemotherapy
with an agent that has good efficacy and
doesn’t affect their quality of life to a
major degree.
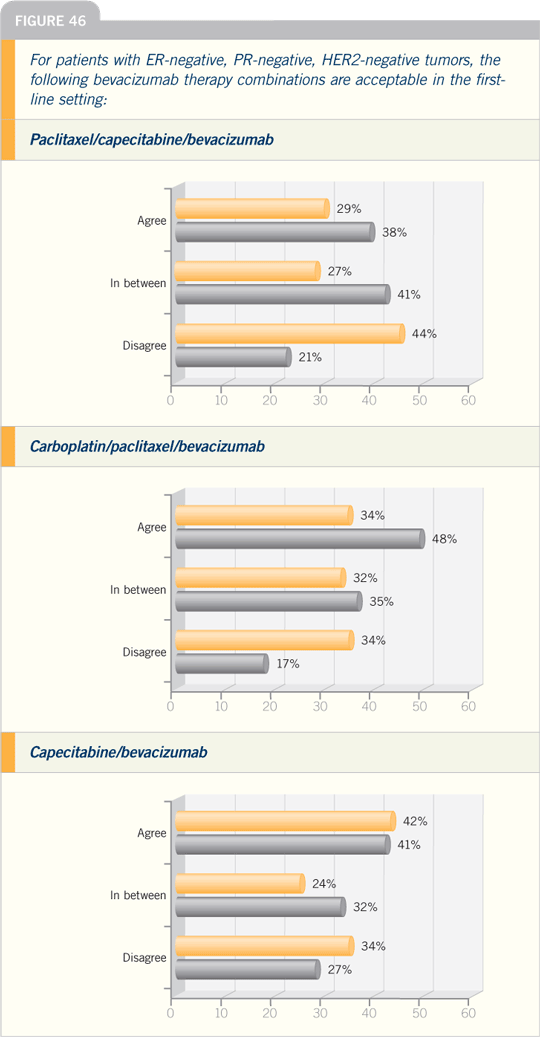
Breast Cancer Update 2005 (8)
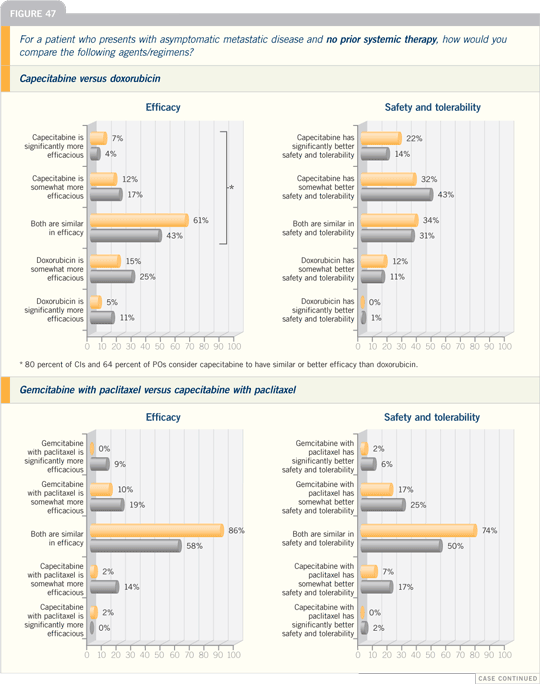
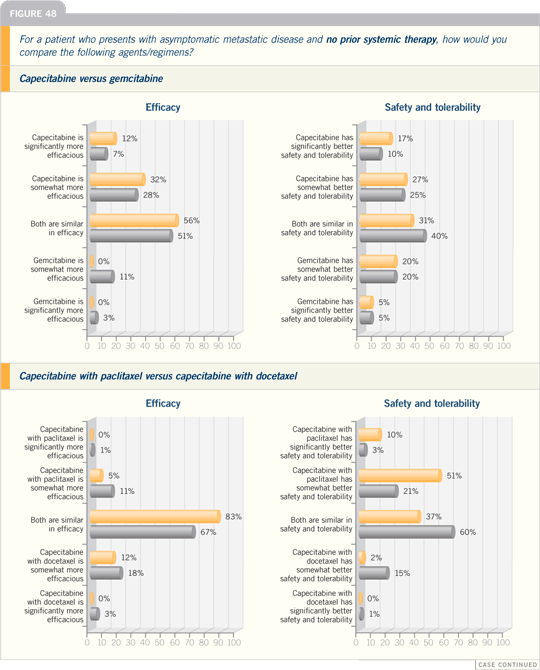
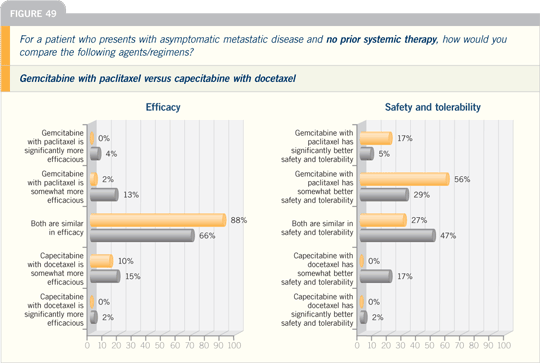
DR VICENTE VALERO: There are two
combination regimens that have proved
to be superior to single-agent taxane
therapy for metastatic disease. One is
gemcitabine with paclitaxel, which was
compared to paclitaxel alone. The data
were presented at ASCO, showing an
improvement in time to progression and
preliminary evidence of an increase in
overall survival.
The other study compared docetaxel
with capecitabine to docetaxel alone and
also showed a time to progression and
overall survival advantage.
Based on the evidence, both of these
combinations are reasonable for first-line
chemotherapy of metastatic disease.
However, in some patients, sequential
chemotherapy is our preference.
I tend to use more sequential single-agent
chemotherapy, but I believe the
role of combination chemotherapy in
some instances is well documented by
the two studies I just mentioned.
For women who have symptomatic
breast cancer with visceral involvement,
it is essential to have a response to alleviate
the symptoms and improve their
quality of life. For those patients, despite
the enhancement of the adverse events,
I strongly consider combination chemotherapy.
Breast Cancer Update 2006 (6)
DR OSBORNE: In some ways, I believe
nanoparticle albumin-bound (nab)
paclitaxel is a little safer compared to the
other taxanes. I’d also be interested to
see how it does, for example, combined
with trastuzumab for HER2-positive
disease or combined with other chemotherapy
regimens to see if the hint that it
might be better in the metastatic setting
plays out in the adjuvant setting.
The attractive thing about it is that you
don’t have to administer premedication.
For patients who are on this drug for a long
period of time, that’s a big advantage.
Dexamethasone premedication can
cause its own side effects. I haven’t used
nab paclitaxel all that often yet, but I like
it and I’m anxious to see how it’s going to
be incorporated earlier in the management
of the disease.
Breast Cancer Update 2006 (6)
DR HENDERSON: I am enthusiastic
about nab paclitaxel. I have a bias in that
I was very involved in the development
of doxorubicin HCL liposome injection
and it, like nab paclitaxel, has a delivery
system that increases the amount of drug
that actually reaches the tumor.
The issue of dose of chemotherapy has
been a complicated one in cancer. When
we examine dose in animal models, we
clearly see a dose effect, and in leukemia
we see an advantage with higher doses.
Almost every oncologist has been taught
as part of his or her earliest training that
dose is a critical factor.
However, in most dose studies it’s
difficult to demonstrate that dose makes
a lot of difference, high-dose chemotherapy
in bone marrow transplant being
a case in point. I believe the reason we
have been unable to show that dose is so
important is that we are examining the
dose we administer rather than the dose
that reaches the tumor.
With a delivery system, you change
the distribution of drug so that less goes
to the normal tissue and more — a higher
dose — reaches the tumor itself. That’s
what happens with doxorubicin HCL
liposome injection and nab paclitaxel. In
both cases we can show that elegantly
in preclinical models. Showing that in
the human, of course, is more difficult
because it’s not so easy to biopsy a tumor
and measure the drug level.
We know that we can administer
higher doses. In CALGB-9342, which
studied paclitaxel doses of 175 mg/m2,
210 mg/m2 and 250 mg/m2 in patients
with metastatic breast cancer, we saw
no significant effect from escalating the
paclitaxel dose. However, there was some
marginal effect from the higher doses
and a suggestion of a longer time to
tumor progression.
In fact, some of the analyses reached
statistical significance as an endpoint. I
believe with nab paclitaxel we are seeing
that we can give higher doses and that
patients tolerate higher doses.
In the preclinical models, mice tolerate
higher doses of nab paclitaxel than
paclitaxel delivered in Cremophor®. In
addition, because of the way the albumin
interacts with the paclitaxel, higher doses
were delivered to the tumor. I believe that’s
why they were able to show a significantly
better outcome with nab paclitaxel. It’s an
interesting step forward.
Breast Cancer Update 2006 (3)
DR GRADISHAR: In terms of first-line taxanes in the metastatic setting, the
data are still more abundant with both
paclitaxel and docetaxel than with nab paclitaxel, so if basing a decision on the
length of experience, those agents have
been around for a longer time.
Next page
|
