| |
Systemic Therapy for Metastatic Disease (continue) |

However, I see no reason to believe
that nab paclitaxel will prove inferior to
those drugs with more data. I believe nab paclitaxel will compare favorably, if not
prove to be superior.
When you examine clinical trials that
have evaluated docetaxel or paclitaxel in
similar patient populations with metastatic
disease, the indirect evidence shows
the activity of nab paclitaxel to be comparable
to docetaxel. These agents may have
similar antitumor effects, so one should
consider other factors, including toxicities,
patient convenience and cost.
If nab paclitaxel can offer the same antitumor
effect as docetaxel and paclitaxel
along with advantages in terms of lack of
premedication and shorter infusion time,
whether or not it would become the preferred
agent is an important question. When you think of busy office practices,
the throughput of patients and convenience
to patients are important. An
upside to nab paclitaxel clearly is the shorter
infusion time and the lack of need for
premedication.
As for the higher acquisition cost of nab paclitaxel, economic analyses suggest
that some of the downstream expenses
related to administering paclitaxel or
docetaxel — specifically the costs of premedications
and antibiotics or growth
factors to manage the neutropenias or
cytopenias — result in a net savings with
the use of nab paclitaxel.
Although we need more information,
I believe we shouldn’t necessarily be put
off by the up-front cost; we should take
into account the whole package of managing
the patient’s treatment.
Breast Cancer Update 2006 (8)
DR LIVINGSTON: Let’s assume nab paclitaxel and paclitaxel are equivalent.
Should we, therefore, simply substitute nab paclitaxel for paclitaxel? We have a
fair amount of data, both from preclinical
systems and from clinical trials,
to suggest that the drug is superior to
paclitaxel, independent of its ability to
prevent allergic reactions.
A reputable randomized study was published
in the Journal of Clinical Oncology that
compared nab paclitaxel to paclitaxel on
an every three-week schedule for women
with metastatic breast cancer. That study
shows a magnitude of difference in terms
of response rate and time to progression,
which is fairly similar to the magnitude
of difference that was demonstrated in
ECOG-E2100 between paclitaxel alone
and paclitaxel with bevacizumab.
However, the paclitaxel with bevacizumab
trial was accepted with great
enthusiasm — legitimately — and presented
in a fairly frenzied special oral
session at ASCO, while the trial involving nab paclitaxel versus paclitaxel was
basically disregarded.
A plausible hypothesis is that nab paclitaxel, in conjunction with other
treatment, could produce a higher
pathologic complete response rate than
standard paclitaxel. This question is
worth answering and may be answered
more expeditiously in the setting of
neoadjuvant therapy, where the pathologic
complete response endpoint can be
obtained quickly, rather than in an adjuvant
trial setting, where it will require
many years to obtain an answer.
In my own practice, I’m prescribing
patients paclitaxel because of the cost differential.
If cost were not an issue, I would
stop administering paclitaxel today and
substitute it with nab paclitaxel.
Interview, September 2006
DR GRALOW: SWOG-S0226 is a
randomized, first-line metastatic study
in which all patients receive an aromatase
inhibitor, and half of them will receive
fulvestrant concurrently.
The group that is randomly assigned
to receive the aromatase inhibitor alone
is asked to switch to fulvestrant at the
time of progression, although we know
we can’t force their next-line therapy.
So it’s a question of an up-front
aromatase inhibitor with a selective estrogen
receptor downregulator (SERD),
fulvestrant, versus an aromatase inhibitor
followed by the SERD. We’re hoping
that we’ll obtain complete estrogen
blockade by using this regimen.
We know that in the ATAC trial,
the anastrozole/tamoxifen combination
arm did not appear to be any better than
tamoxifen alone and certainly wasn’t
going to be the superior arm.
Tamoxifen can have some proestrogenic properties in an otherwise depleted
estrogen state. Fulvestrant shouldn’t have
these. It’s a pure antiestrogen and thus is
an interesting concept that is different
from considering an aromatase inhibitor
with or without tamoxifen. Certainly,
preclinical data suggest that this could
work. It makes sense, and we have high
hopes that it could be better.
Breast Cancer Update CME Meeting
June 2005
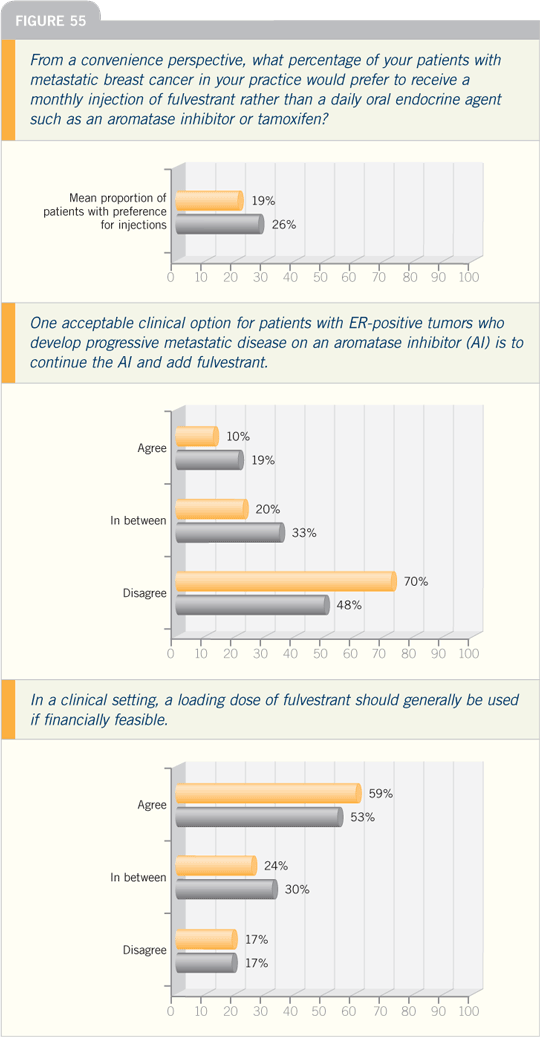
DR OSBORNE: In the clinical setting, I
believe it is a good idea for patients who
are progressing on an aromatase inhibitor
to continue with an aromatase inhibitor
and add fulvestrant, but we have no
data. I have done this with a few patients
based on two preclinical studies that
have evaluated this: my own and Angela
Brody’s.
Fulvestrant seems to work much better
when there’s no estrogen around.
Even though postmenopausal women
have lower estrogen levels in the blood,
their tumors don’t necessarily have lower
estrogen levels, and fulvestrant seems to
be more effective when estrogen is low.
In patients progressing on tamoxifen,
tamoxifen binds the estrogen receptors
and may actually stimulate growth of the
tumor — it certainly is no longer inhibiting
it. Treating these patients with an
aromatase inhibitor will be ineffective
until all the tamoxifen is gone, which
takes a couple of months. Fulvestrant, on
the other hand, competes with tamoxifen
for binding, thus the response may be
quicker with fulvestrant than with an
aromatase inhibitor in that setting.
Breast Cancer Update 2005 (9)
DR CHARLES L VOGEL: Fulvestrant is a
very good drug that has minimal toxicity.
We don’t even encounter much in the
way of buttock pain with a five-cc injection.
We’re also not seeing the degree
of joint discomfort that we see with the
aromatase inhibitors.
In terms of efficacy, fulvestrant seems
to be equivalent to anastrozole. Based on
data published this year in Cancer, there
seems to be no difference in overall survival
in the randomized trials of anastrozole
versus fulvestrant. Fulvestrant is a good
drug and a viable alternative to aromatase
inhibitors in patients who have disease
progression on tamoxifen.
We do have to contend with the
randomized trial of fulvestrant versus
tamoxifen, where we expected a strongly
beneficial effect for fulvestrant over
tamoxifen, which was not forthcoming.
There were some subsets where
fulvestrant appeared to be better, but the
overall results were about the same.
Breast Cancer Update 2005 (4)
DR GRADISHAR: An important issue is
whether fulvestrant at 250 milligrams is
optimal, even though that’s the approved
dose. Some of the data, including preclinical
data generated by Kent Osborne and
others, suggest that this dose is on the
low end of the curve where you might
expect the optimal response rate.
Although we may be able to increase
the dose, administering 250 milligrams
in each buttock, doing that too frequently
becomes prohibitive, and patients may
not tolerate it.
Some strategies have evaluated quickly
increasing serum levels of fulvestrant,
and those strategies have included administering
loading doses of 500 milligrams
and then, within two weeks, administering
another 250 milligrams and then
proceeding to the monthly schedule.
Those strategies are based on mathematical
modeling that have shown an
ability to achieve steady-state levels much
quicker and, consequently, achieve a biologically
relevant dose of drug circulating
in a given patient much faster.
Breast Cancer Update 2005 (4)
DR OSBORNE: We expected fulvestrant
to be superior to tamoxifen, but in the
first-line setting it proved to be similar,
not better. That’s peculiar because
second-line trials show fulvestrant to be
equal to or better than aromatase inhibitors,
and aromatase inhibitors have been
shown to be superior to tamoxifen.
It may be that we’re not dosing
fulvestrant correctly. We know from the
randomized trial that half of the currently
recommended dose is insufficient, and
we know it takes three to six treatments
to achieve steady state blood levels with
fulvestrant, so perhaps a higher dose or a
loading dose (or both) is required. These
options are being investigated.
Breast Cancer Update 2004 (3)
DR JOHN F R ROBERTSON: Fulvestrant
at 250 milligrams is an effective dose, as
demonstrated by the clinical trials. It is as
effective as anastrozole as second-line therapy
and equivalent to tamoxifen as first-line
therapy in postmenopausal women.
In premenopausal women, data suggest
that 250 milligrams of fulvestrant
is not effective at down-regulating the
estrogen receptor. This raises questions
about whether a 250-mg dose of
fulvestrant leads to complete down-regulation
of the estrogen receptor in postmenopausal
women. Could a higher dose
of fulvestrant achieve more?
Two strategies exist to increase the
dose of fulvestrant. The first is a loading
dose sequence. The second is the administration of a higher dose of fulvestrant.
For example, instead of administering
one 5-mL injection every month in one
buttock, one might administer one 5-mL injection in each buttock, for a total
of 500 milligrams. Future studies are
needed to determine the dose-response
curve for fulvestrant.
Breast Cancer Update 2004 (9)
DR GABRIEL N HORTOBAGYI: I believe
the trials of fulvestrant underestimate
the efficacy of this agent. The dosing
schedule used was probably too low
because by the time steady state was
reached, many patients were off study,
presumably because of progression.
In my group, we administer loading
doses of 500 milligrams of fulvestrant,
followed by 500 milligrams two weeks
later and then 250 milligrams monthly.
The pharmacokinetics of fulvestrant
suggest a loading dose would be beneficial,
so it concerns me that the comparison
of fulvestrant to anastrozole in
a tamoxifen-resistant population might
not have revealed the true efficacy of
fulvestrant. It showed fulvestrant to be
at least as effective as anastrozole, but I
expected it to be superior. We may need
to repeat some of these studies with a
more appropriate dosing schedule.
Breast Cancer Update 2003 (6)
DR O’SHAUGHNESSY: I am a little
disquieted by the fact that it can take
three to five months to reach a steady
state with fulvestrant.
A patient with rapidly progressing
disease may not benefit from fulvestrant,
but fortunately most women with hormone-responsive breast cancer have relatively
indolent disease. I’m interested in
the clinical trial in which they are loading
fulvestrant at 500 milligrams every
two weeks for a couple of doses and then
reducing it to 250 milligrams monthly.
That makes sense to me.
Breast Cancer Update 2005 (8)
DR VALERO: At MD Anderson, we use
a loading dose of fulvestrant. We administer
500 milligrams on day one, 250
milligrams on day 15 and day 29 and
then monthly.
Many of the key investigators in the
early development of the drug believe it is
important to attain steady state, but we
have no randomized data for the loading
approach. Currently, it is FDA approved
at 250 milligrams monthly and is reimbursed
by Medicare at that dose.
With all of those caveats, I believe
— and I don’t know if this is my bias
— the loading approach is reasonable.
However, although we think that may be
the best dosing schedule, we won’t know
unless we do a pharmacokinetic study to
show that the doses are equally effective.
Breast Cancer Update 2006 (7)
DR CARLSON: I believe a loading dose
of fulvestrant should generally be used
in clinical practice, and I continue to see
an increase in the number of patients
treated with fulvestrant. That’s reasonable,
and experience has confirmed the
tolerability of the drug and the efficacy
of the therapy. My expectation is
we’ll see nothing but increased use of
fulvestrant.
In terms of use for the premenopausal
woman, I believe that in the metastatic
setting, we will see increasing numbers
of patients treated with fulvestrant after
they are put in a menopausal state.
In part this is because I believe the
truly limited number of endocrine agents
we have available for the treatment of
premenopausal breast cancer means that,
functionally, after a premenopausal woman
has been treated with tamoxifen, you’re
obligated to make her postmenopausal.
Once she’s postmenopausal, the whole
spectrum of endocrine agents, which are
effective in the postmenopausal woman,
become available.
Because my expectation is that the
women will be on hormone therapy for
some length of time, I often send those
women to the gynecologic oncologist for
a laparoscopic oophorectomy.
Dodwell D, Vergote I. A comparison
of fulvestrant and the third-generation
aromatase inhibitors in the second-line
treatment of postmenopausal women
with advanced breast cancer. Cancer Treat Rev 2005;31(4):274-82.
Fulvestrant is the first antioestrogen to
demonstrate efficacy in tamoxifen-resistant
disease, highlighting the difference
in mode of action between fulvestrant
and the SERMs (which show only limited
efficacy in this setting). In phase III
studies, fulvestrant was at least as effective
as anastrozole in terms of clinical
efficacy and was well tolerated.
Furthermore, fulvestrant is associated
with significantly fewer joint disorders
(arthralgia, arthrosis and arthritis) compared
with anastrozole. Indirect comparisons
suggest that fulvestrant also
offers comparable efficacy to letrozole
and exemestane, and may have some tolerability
benefits over these agents in the
second-line treatment of postmenopausal
women with advanced breast cancer.
Breast Cancer Update 2006 (3)
DR GRADISHAR: The SoFEA trial is
evaluating the use of endocrine therapy
in the metastatic disease setting,
comparing exemestane as a single
agent to fulvestrant to the combination
of anastrozole and fulvestrant. The
combined therapy arm may be the most
interesting one.
The rationale behind it is not only
removing the ligand for the receptor —
which is what the aromatase inhibitor
would do by decreasing the amount of
circulating estrogen — but also eradicating
the actual target, which is the receptor.
Answering whether absolute removal
of those two targets will result in a better
outcome is one of the goals of the study.
Breast Cancer Update 2006 (3)
DR ROBERTSON: In cell culture, when
MCF7 cells are depleted of estradiol,
they become extremely sensitive to low
levels of estrogen. The cell line can be
inhibited if fulvestrant is then titrated
into that long-term estrogen-deprived
cell line.
The rationale behind the SoFEA study
is that the development of resistance to
aromatase inhibitors may result from an
increased sensitivity of breast cancer cells
to very low levels of estradiol.
Fulvestrant competes with estradiol
for the estrogen receptor on a one-toone
basis, so that upon progression
while on the aromatase inhibitor, the
addition of fulvestrant to the aromatase
inhibitor might result in a better blocking
effect. I hope the SoFEA trial will
show that improvement occurs from
the combination of fulvestrant and an
aromatase inhibitor.
This will be an interesting study, not
only because it will tell us what to do in
second- or third-line therapy but because
it will also tell us about mechanisms of
action and whether they are important
in breast cancer.
Stephen R Johnston et al. Life following
aromatase inhibitors — Where now for
endocrine sequencing? Breast Cancer Res
Treat 2005;93(Suppl 1):19-25.
Fulvestrant (‘Faslodex’) is a new ER
antagonist with no agonist effects that
binds, blocks and degrades the ER. Due
to its unique mode of action and lack of
cross-resistance with existing treatments,
fulvestrant is an effective therapeutic
agent for use in sequential endocrine
regimens. Fulvestrant has established
efficacy in tamoxifen-resistant disease
and there is a growing body of evidence
demonstrating its efficacy in patients
with AI-resistant disease.
In preclinical models, MCF-7 cells
undergoing LTED are refractory to
tamoxifen but sensitive to fulvestrant,
suggesting fulvestrant is a more appropriate
choice following AI resistance.
The steroidal AI, exemestane is also
an option in nonsteroidal AI-resistant
disease. Clinical trials are underway to
compare fulvestrant with exemestane as
an appropriate therapy following the
onset of AI resistance.
Select publications
|
