| |
Treatment of Patients with HER2-Positive Disease |

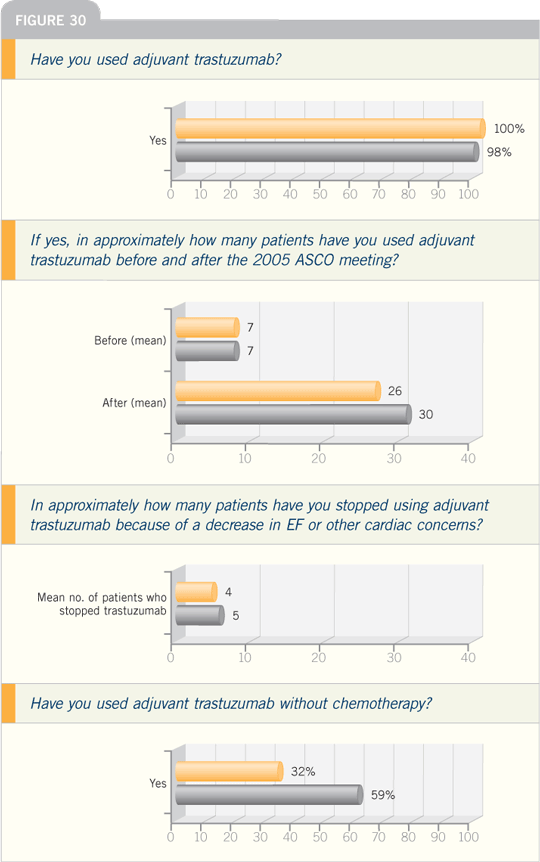
Breast Cancer Update 2006 (6)
DR CHARLES E GEYER JR: The exciting
thing about the adjuvant trastuzumab
data has been that no matter how you use
it, patients derive a substantial benefit.
Small differences probably occur among
the different ways of using trastuzumab,
which we can’t definitively address because
the trials weren’t designed that way, but
it’s clear that trastuzumab is the most
important element of therapy for a patient
with HER2-positive breast cancer.
TCH (docetaxel/carboplatin/trastuzumab)
certainly has low cardiac toxicity,
but TCH is not a gentle regimen for
an elderly woman.
I believe the weekly carboplatin/paclitaxel/trastuzumab that we use for
metastatic disease is active and well tolerated.
Those are the substitutions I
believe would be reasonable to consider
for an elderly patient, if you felt you
needed to use chemotherapy.
Can you use trastuzumab alone or
with hormone therapy? I’m sure you can.
You have to use your clinical judgment.
Trastuzumab is active without chemotherapy;
there is no question about that,
but if I were going to use trastuzumab, I
would like to use some kind of chemotherapy,
maybe just four cycles á la the
HERA trial.
Breast Cancer Update 2006 (7)
DR TRIPATHY: Theoretically, adjuvant
trastuzumab monotherapy may be a
reasonable approach. Remember that in
the HERA study, a 50 percent reduction
in recurrence was seen in all patient
groups, which included all comers. But
keep in mind that as a requirement of the
HERA study, all patients received prior
chemotherapy. We know that synergy
exists between chemotherapy and
trastuzumab, so we could argue that
trastuzumab works best in the context of
chemotherapy. Although I would guess
that trastuzumab monotherapy would
reduce recurrence, we don’t have any data
to support that. Sometimes extrapolations
require too much speculation, and
I believe the leap to trastuzumab monotherapy
is one of those situations.
Trastuzumab monotherapy would be
good to include in a trial if we could
identify an appropriate patient population. We currently have options for chemotherapy
regimens that are nontoxic,
like some of those used in the HERA trial. Dr Heikki Joensuu has studied
vinorelbine followed by FEC, opening
the door to studies of agents with pre-clinical
synergy and great activity in the
advanced setting. I would advocate a trial,
maybe with vinorelbine and trastuzumab
in one arm and trastuzumab alone in
another arm.
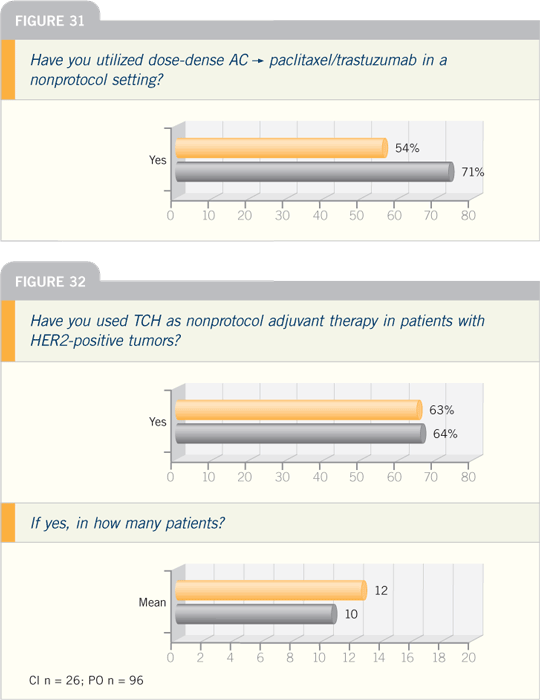
Combining a taxane alone with
trastuzumab is a little more reasonable,
although again, we do not have the data.
Technically, the HERA study would have
allowed that, but I don’t think there were
any patients who received paclitaxel alone.
In talking about where one would draw
the line, a taxane alone with trastuzumab,
in my mind, would be reasonable.
Breast Cancer Update 2006 (7)
DR PEGRAM: If you’re going to consider
an anthracycline-based adjuvant regimen
followed by trastuzumab with
taxanes, you need to tell patients that it
carries a defined risk of cardiotoxicity. In
particular, in the NSABP-B-31 adjuvant
trastuzumab trial, after four cycles of
AC approximately four to five percent of
the patients were ineligible for adjuvant
trastuzumab at all. In clinical practice,
it is important to measure the ejection
fraction before and after the AC to make
sure that your patient would have met
the eligibility for the study and you could
draw on that safety database.
Moreover, during the year of adjuvant
trastuzumab for the patients who
received the drug, an additional approximately
15 percent of the patients had to
drop out because of decreases in ejection
fraction, which I find alarming. My fear
is that in the community, busy practitioners
will forget to obtain those ECHOs
and MUGAs every three months,
which was done on all of the adjuvant
trastuzumab trials.
I’m fearful of what might happen for
patients who have marked decreases in
ejection fraction but may not be having
symptoms from heart failure yet, and
because they didn’t get their ECHO or
MUGA they are simply continued on
more trastuzumab. Clinicians need to
know that if they’re going to prescribe
adjuvant trastuzumab, they should do
so following the same guidelines that
were used in those protocols, which was
an ejection fraction assessment every
three months during the one year of
trastuzumab.
If the ejection fraction decreased to
less than institutional norms, patients
had to drop out. If it dropped 15 points
and was above institutional norms, they
had to hold the trastuzumab, at least
temporarily, and wait for recovery. If
recovery was evident on a follow-up one
month later, then they were allowed
to attempt to reinstitute it, as long as
they were not symptomatic or at lower
than institutional norms. These protocol
guidelines are available, and they should
be strictly followed if you’re going to use
anthracyclines.
Breast Cancer Update 2006 (6)
DR GEYER: For me, the precedent for
cardiac monitoring of a patient receiving
trastuzumab has been set by the
adjuvant trials. The plan was a reasonable
one: Check imaging halfway through
the chemotherapy, check it at the end of
chemotherapy and then check it three
months later. It made sense for the trial,
and I believe it makes sense for the clinic.
In NSABP-B-31 and NCCTG-N9831,
we stopped the drug in a significant
number of patients — about 15
percent of the patients had asymptomatic
declines in LVEF. We don’t know that we would have seen a higher rate of clinical
heart failure if we had continued to treat
them, but it’s a reasonable assumption.
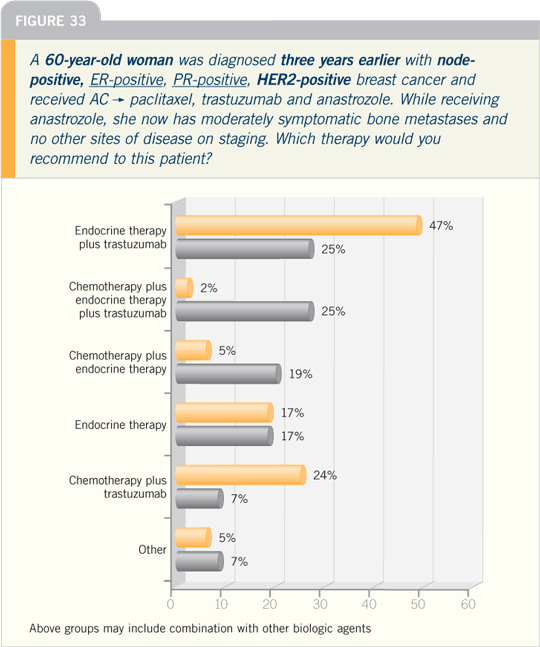
Breast Cancer Update 2006 (8)
DR BURSTEIN: The biggest question I
get at tumor boards right now is how
to approach patients who have small
HER2-positive tumors — patients
who wouldn’t have been eligible for the
adjuvant trials, such as the patient with
the 7-mm, ER-negative, HER2-positive
tumor or the 1.2-cm, ER-positive,
HER2-positive tumor.
We don’t have great data on the outcomes
for these women. We have proposed,
and I believe we’ll put forward, a
multicenter trial evaluating trastuzumab
with paclitaxel as a treatment regimen for
patients at low risk. We will treat something
in the order of 300-400 patients
in what will essentially be a feasibility
study to show that if you carefully select
the patients at low risk and administer a
paclitaxel/trastuzumab combination that
should be well tolerated, you have a low
risk of recurrence.
We would love to see a huge randomized
trial for these women, but that is
impractical given the resources and the
generally low risk for patients with node-negative
disease.
Breast Cancer Update 2006 (8)
DR RAVDIN: Currently, the Adjuvant!
program doesn’t make projections for
trastuzumab outcomes at 10 years
because we have data with follow-up of
only two to three years. In general, many
of the patients with ER-positive disease
will experience recurrence later. If we
don’t know that part of the story, we
could give wildly inaccurate estimates.
Instead, the program provides a separate
output for trastuzumab, projecting
benefit at five years, which is reasonable
to talk about.
Some patients have been followed for
five years in the trastuzumab trials.
The program also provides information
about some of the toxicities and
uncertainties about toxicity. Version 9
of the breast cancer program is about to
be released. For the first time, it includes
HER2 status as one of the program
parameters.
Breast Cancer Update 2006 (7)
DR PEGRAM: Fulvestrant, rather than
tamoxifen or the aromatase inhibitors,
makes the most sense to combine with
trastuzumab because in HER2-positive
breast tumor cells there is ligand-independent
activation of the estrogen
receptor. That is, the cross talk between
HER2 signaling and the estrogen receptor
can activate estrogen-dependent
genes in the absence of estradiol.
The aromatase inhibitors remove the
ligand for the ER, but the ER can still be
turned on by HER2 signaling. So that’s
a strike against aromatase inhibitors.
Tamoxifen can also be more agonistic as
a result of this cross talk mechanism.
The question is, how can you tackle
such a complex issue? It would be ideal
to eliminate the estrogen receptor, and
that’s exactly what fulvestrant does.
Therefore, it is appealing from a theoretical
point of view to incorporate
HER2-directed therapy with fulvestrant,
and we have a randomized Phase II trial
under way in the metastatic setting comparing
fulvestrant alone to trastuzumab
alone to the combination. It’s accruing
slowly, unfortunately, and may have to
be pared down to get some point estimate
on the activity of the combination
in the future.
I have a number of patients on
fulvestrant and trastuzumab who are
doing well, although they were started
on the treatment off protocol because our
protocol wasn’t open when they started.
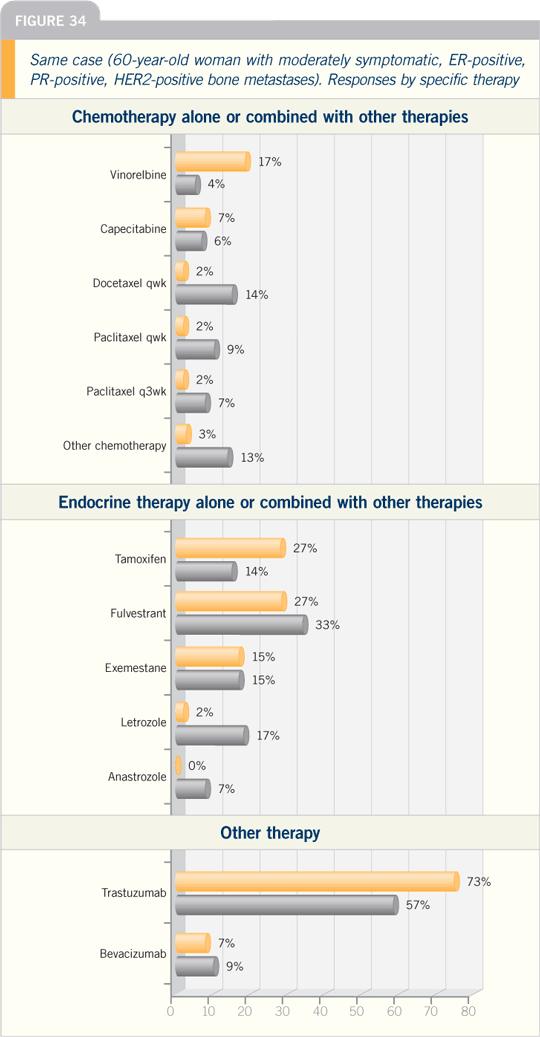
I’ve had some nice anecdotal responders
on that combination. Remember
that many of these patients have already
received adjuvant aromatase inhibitors
anyway. So fulvestrant is a reasonable
consideration when they relapse.
Breast Cancer Update 2006 (7)
DR TRIPATHY: The patient with HER2-positive disease who was treated six
months or a couple of years ago poses a
dilemma. You have to decide one way or
the other — if the patient comes to you,
then you can’t just throw up your arms
and say you don’t know. My approach is
to individualize therapy.
We know that in both the HERA
study and the North American studies,
the hazard rate in the entire population
was still pretty high at two and
three years — around 10 percent per
year. Now, the question is, does the risk
reduction still apply two years out? That
we don’t know.
I can make an analogy with hormonal
therapy. I was surprised when the data
came out for patients who had been on
tamoxifen for five years and were then randomly
assigned to placebo versus letrozole.
Even when initiating hormonal therapy
after five years, approximately a 40 percent
reduction was still evident, which is about
what we expect of hormonal therapy anyway.
So at least in the case of hormonal
therapy, it looks as though the odds reduction
is preserved whether treatment is
given up front or much later.
Extending that to trastuzumab,
patients at average risk would still have
an annual reduction in hazard ratio of
about five percent per year. So that would
be 10 percent over two years and maybe
even more as time goes on. We have to
realize that even two or three years out,
an odds reduction is likely. Again, this
is where you need to tailor treatment.
For a patient with node-negative disease
who is a borderline candidate, I would
use trastuzumab up front or maybe six
months out. For patients with two or
three nodes, I believe it’s appropriate to
consider trastuzumab even two years
out. I know that’s a stretch, but at least it is based on data on annual hazards and
some extrapolations of the activities of
other drugs.
I believe it’s reasonable even when
the patient was treated more than two
years ago. We don’t have hazard rates
that far out. Right now, we have them
as far as three years on the longest-running
NSABP study. Keep in mind that
every year we will have more data on the
annual hazards. Currently I would say
two, two and a half years is my limit. But
a year from now, when we will have more
data, I believe we can feel more comfortable.
So it’s a moving target, and we have
to stay tuned.
Breast Cancer Update 2006 (8)
DR BURSTEIN: With the tremendous
outpouring of the major adjuvant
trastuzumab trials in 2005, a lot of retrospective
clean-up work has begun. People
want to see if they can figure out which
tumors benefited most markedly from
trastuzumab. Is there a marker — whether
it’s cMYC or TOPO II — or do we have
something else that will predict which
patients do or don’t need trastuzumab?
Which patients who receive trastuzumab
have such a fabulous prognosis that they
don’t need anything else?
In terms of treatment, the next big
trial will be from the Breast International
Group (BIG). This will be a four-arm
randomized trial of trastuzumab (BIG
2-06) for patients who have HER2-positive breast cancer and have received
chemotherapy. Patients will receive
trastuzumab versus lapatinib versus a
combination of the two versus a sequential
treatment program of trastuzumab
followed by lapatinib. Some patients will
receive only lapatinib.
Another controversy is that this study
follows the HERA treatment program,
in which patients would, for the most
part, receive chemotherapy first and then
receive the biological therapy with the
option of receiving the biological therapy
concurrently with taxane therapy. I have
been impressed that the best results seen
with trastuzumab in the adjuvant setting
and with trastuzumab and lapatinib in the metastatic setting occur when you
pair these products with chemotherapy.
By not insisting on administering these
drugs with chemotherapy, you probably
do not optimize the beneficial effects
of these drugs, and that is a substantial
criticism of the study.
Additionally, we have an awful lot of
good-quality data on trastuzumab but
not every patient will receive trastuzumab,
and that will affect accrual in some quarters.
A lot of excitement has arisen about
lapatinib, but I believe that is a potential
weakness of the study design. You have to
bring new agents forward and you need
corporate sponsorship for trials, so hard
choices have to be made, but I believe that
this will affect some patients’ and doctors’
willingness to contribute to that study.
Breast Cancer Update 2006 (6)
DR HENDERSON: TOPO II makes sense
scientifically. We began talking about
it more than a decade ago. It’s particularly
interesting because TOPO II is on
the same chromosome as HER2, and
in the early papers we thought there
was a correlation between the impact of
doxorubicin and HER2.
I don’t believe that has really held up.
Certainly, when Dan Hayes presented
the data from CALGB-9344 at ASCO
2006 we didn’t see a correlation between
HER2 expression and doxorubicin dose.
I believe anthracyclines are so powerful
and so valuable in the treatment of
breast cancer that I would be hesitant to
leave out doxorubicin until we had compelling
data that a particular group of
patients received no benefit from it.
It’s similar to the way we view estrogen
receptor status and chemotherapy.
We know that patients with ER-positive
disease derive less benefit from chemotherapy
than those with ER-negative
breast cancer, but it’s not an all-or-none
phenomenon.
I believe the same principle applies
here. When will you be comfortable
enough to leave out a powerful drug? As
good as the taxanes are — and I am enthusiastic
about them — I don’t believe they
are any better than the anthracyclines in
the treatment of breast cancer.
Breast Cancer Update 2006 (6)
DR LIPPMAN: The data on TOPO II
that were presented at the San Antonio
Breast Cancer Symposium were very
exciting, and I hope they are substantiated.
It makes biological sense — TOPO
II is a target for doxorubicin. That would
potentially explain which subsets of
patients gained particular advantage from
the doxorubicin combinations compared
to the platinum combinations.
I’m not ready to draw the conclusion
that Professor Slamon seemed to want to
draw, which is that in those patients who
did not overexpress TOPO II, the use of
a nondoxorubicin-containing combination
was as efficacious.
That may be true, but I’m not there
yet. I believe we need more analysis.
Given the additional cardiac risks of using
trastuzumab with doxorubicin, particularly
in older women, it would be nice to
have a less cardiotoxic regimen to use.
In that same regard, I found the
data Soonmyung Paik presented from
the NSABP on cMYC overexpression
extremely exciting and, once again, biologically
plausible.
cMYC is an oncogene that is generally
upregulated when cells are stimulated to
grow; it is part of the growth response, and
it is clearly overexpressed in about 20 to 25
percent of human breast cancer cases.
The question is, why is it that many
patients with tumors that unquestionably
overexpress HER2 do not respond to
trastuzumab? Even in previously untreated
patients, the response rates are only
about 35 percent.
Dr Paik’s data showed rather conclusively
that only in those patients whose
tumors coexpressed cMYC and HER2
was a response to trastuzumab seen.
Those data must be replicated, but if that
were the case, this observation would be
tremendously insightful.
Breast Cancer Update 2006 (8)
DR JENNY C CHANG: The bottom-line,
take-home message from Soon Paik’s
data was that if you have HER2-positive
and cMYC-positive disease, you do
very well with trastuzumab-based therapies. cMYC is an oncogene, and it was
expected that if you had cMYC-positive
disease, you would do badly.
In the adjuvant trastuzumab study,
however, patients with cMYC-positive
disease who received trastuzumab did
extremely well. Their chance of relapsing
was low — less than 10 percent, which
was counterintuitive.
TOPO II is a different story. TOPO
II is the target for anthracyclines. We
also know trastuzumab in combination
with anthracyclines adversely affects cardiac
function and increases cardiotoxicity.
The BCIRG wanted to determine
whether any subpopulations of patients
receiving trastuzumab could be spared
therapy with anthracyclines.
As presented at the 2005 San
Antonio Breast Cancer Symposium,
the study demonstrated that, across the
board, the nonanthracycline-containing
trastuzumab-based regimen was not
superior to anthracycline-containing
trastuzumab-based therapy. The subset
of patients with TOPO II nonamplified
disease who received a nonanthracycline-containing regimen, however, did
as well as the patients who received
anthracyclines.
Breast Cancer Update 2006 (7)
DR TRIPATHY: In the next generation of
clinical trials in HER2-positive disease,
we’d like to improve the odds reduction.
We would also like to use drugs that
target other aspects of the HER2 pathway.
A leading candidate is lapatinib, a
dual HER1 and HER2 kinase inhibitor
that also inhibits the same target,
HER2, but in a different way.
It works on the cytoplasmic kinase
domain, which is part of the signaling
initiator. Some early data show a higher
response rate when you combine lapatinib
and trastuzumab. We already know from
early pilot trials that previously untreated
patients with HER2-positive disease
show good response rates with lapatinib.
Bevacizumab with trastuzumab is
also a reasonable combination to study. I
would prefer to try to isolate the patients
who will benefit, but without that, I do
believe it’s reasonable. Some pilot studies also show that the bevacizumab/trastuzumab combination is safe and
active. We have no randomized studies
yet, but I believe that would be a reasonable
place to look.
Breast Cancer Update 2006 (8)
DR LISA A CAREY: I consider HER2-driven breast cancer, in a biologic sense, as
being at least two different groups. The
HER2-positive, hormone receptor-negative
group is different from the HER2-positive, hormone receptor-positive group.
They both benefit from HER2-targeted
treatments, but they are different.
In terms of how HER2 functions,
we’re obtaining a lot of information from
the emerging studies of trastuzumab
resistance and the pathways that are
important in trastuzumab resistance.
The first issue — and I believe lapatinib
speaks to this — is whether HER1 is
important in acquired HER2 resistance.
The studies of HER1 expression in
de novo trastuzumab resistance have not
been particularly compelling. They’re
also not very big. The fact that lapatinib
shows efficacy in patients with acquired
trastuzumab resistance, I believe, provides
a strong suggestion that the HER1
pathway may be implicated in getting
around HER2 signaling. Tumor cells
are smart, and they figure out ways to
go around our therapeutic interventions. They co-opt nearby pathways.
One of the ways they co-opt is by using
HER1. Similarly, instead of borrowing a
neighbor to stimulate the same pathway,
they can use a neighboring pathway that
stimulates the same downstream molecules. That’s where the IGF1R data
fit in, which do not so much indicate
co-opting as simply a redundant pathway.
Fortunately, several IGF1R, largely
antibody-based therapies are entering
clinical trials.
Breast Cancer Update 2006 (6)
DR GEYER: We are committed to collaborating
with Dennis Slamon and the
BCIRG jointly on the concept of adding
bevacizumab to adjuvant trastuzumab.
We have been waiting for their pilot
data evaluating the combination of bevacizumab and trastuzumab as frontline
therapy for patients with HER2-positive disease. The trial is progressing
well, and from what they have been able
to share, it looks as if this is something
we definitely will be pursuing.
When patients’ tumors have HER2
amplification, a high percentage —
about three quarters of the patients —
also have upregulation of VEGF. Those
patients do not do well when treated
with chemotherapy alone — they have a
strikingly poor outcome.
The assumption is that something is
mechanistically driving the cancer, and if
you shut down both of those pathways,
you will improve outcomes. Preclinical
models look very strong, and they were
the justification for taking this into a
clinical trial.
We are currently working on a straightforward
concept evaluating trastuzumab
versus lapatinib versus the combination
using an AC followed by weekly
paclitaxel template as neoadjuvant therapy.
All the patients will receive that basic
chemotherapy regimen, and the HER2
blockade will start with paclitaxel.
Then the patients will have surgery
to determine the pathologic complete
response rate. After surgery, all the
patients will receive trastuzumab for one
year. They will be receiving standard therapy
with trastuzumab, but we will obtain
baseline tissue and do the correlative work
to see if we can determine which patients
might do better with each of the drugs
individually or in combination.
Select publications
|
