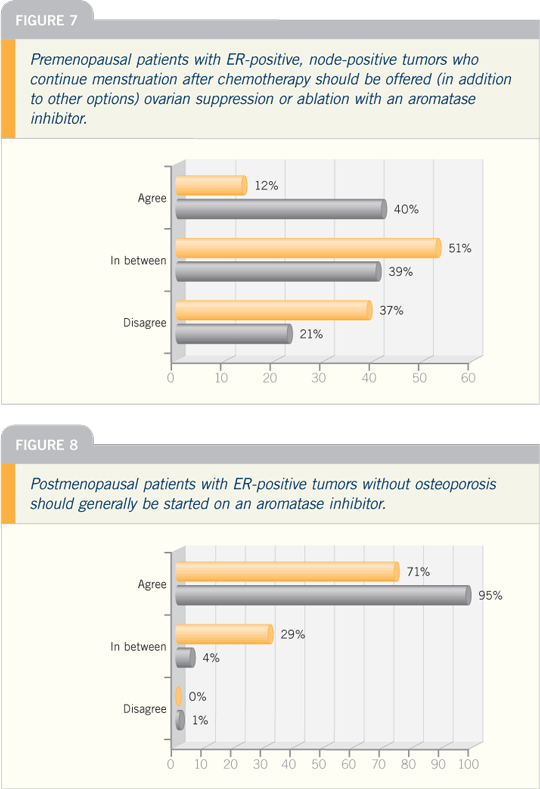
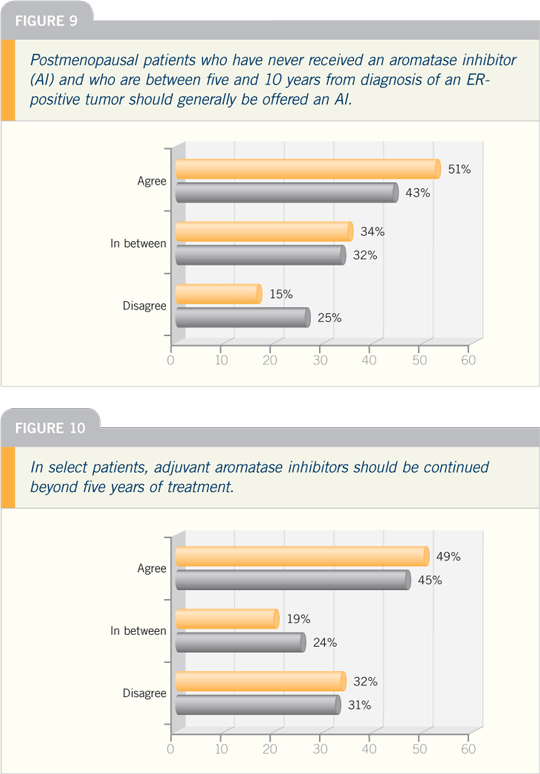
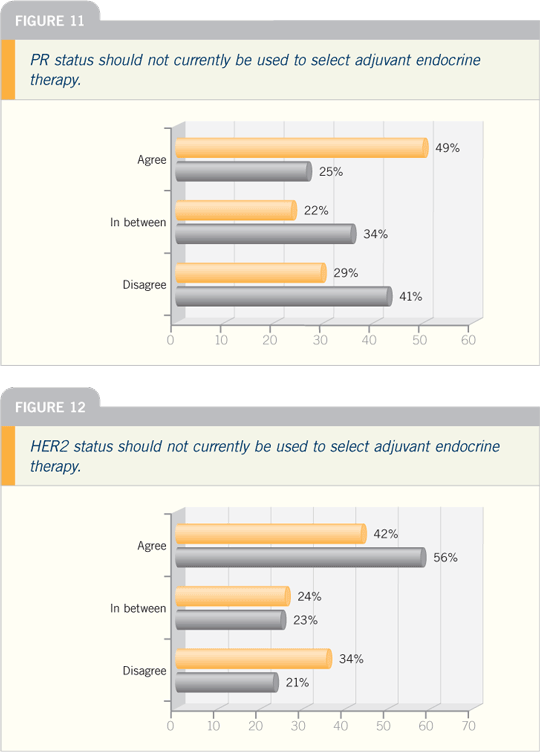
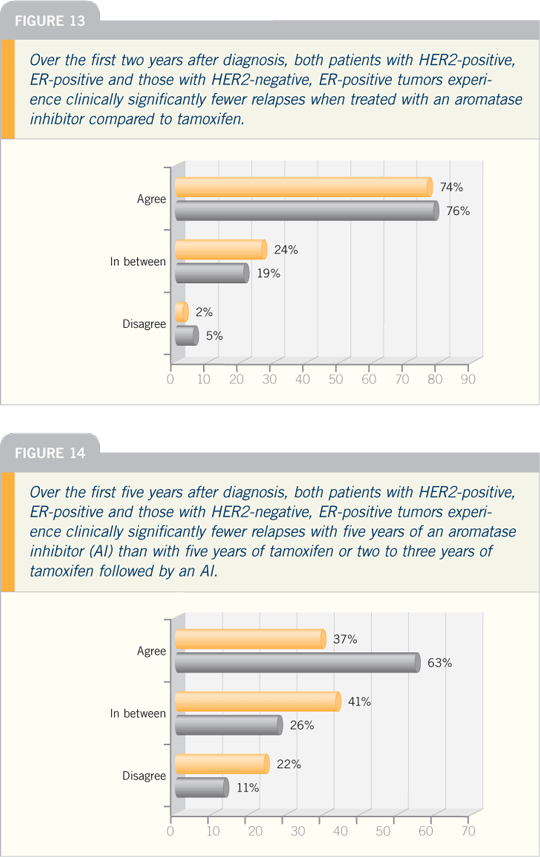
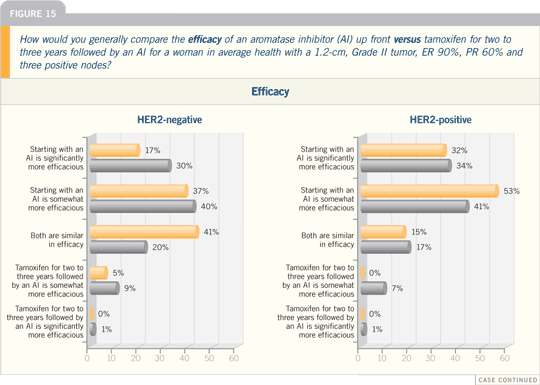
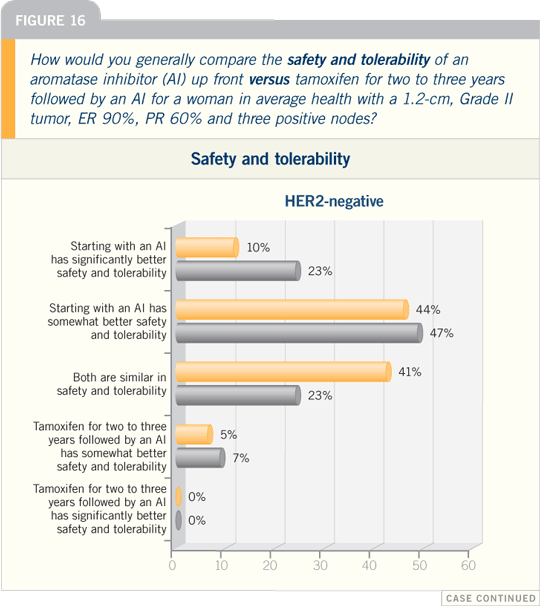
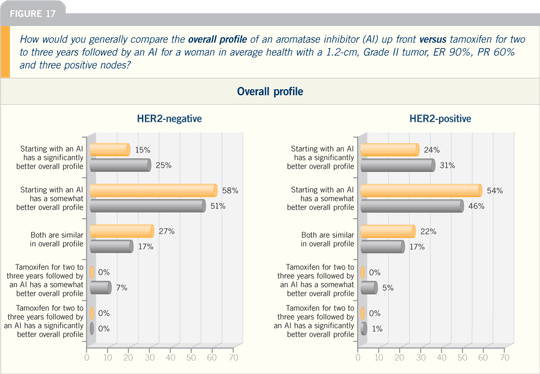
| |
Adjuvant Endocrine Therapy |

Breast Cancer Update 2004 (1)
DR HAROLD J BURSTEIN: The IBCSG
is coordinating a series of three nested
trials: SOFT, PERCHE and TEXT.
These studies address what is probably
the most important conceptual question
in premenopausal breast cancer right
now: Beyond tamoxifen, does planned
ovarian suppression benefit patients? In
particular, does it benefit women who
receive chemotherapy or who don’t receive
chemotherapy, and if a woman experiences
chemotherapy-related amenorrhea,
does she still need ovarian suppression?
These are important trials that offer a
wonderful opportunity for community
oncologists to participate in answering
this critical question. Currently, I consider
ovarian suppression for two groups of
patients. The first group includes patients
at high risk — multiple positive nodes,
very high-risk tumors — and particularly
young women, less than 35 or 40 years
of age, who may not go into menopause
with chemotherapy.
The other group includes women who
are at the opposite end of the spectrum
— very low-risk tumors, smaller tumors,
node-negative — for whom the benefits
of chemotherapy are small. With these
women, I present ovarian suppression as an
option, not necessarily in addition to chemotherapy
but perhaps even instead of it.
Breast Cancer Update 2006 (5)
DR DANIEL F HAYES: I believe an important
issue, which has been lost, is that
all of the aromatase inhibitor studies
enrolled women who were postmenopausal
by virtue of not having a period
for at least a year prior to enrollment. We
have estrogen ablation studies ongoing for
premenopausal women, such as SOFT,
TEXT and PERCHE. We don’t know
the answers from those studies yet.
I believe estrogen ablation is a more
effective therapy than a SERM, but I
also believe it’s more toxic. I’m very supportive
of those trials. We have enrolled
11 patients on SOFT. They’re important
studies, almost as much for the toxicity
as for the outcomes.
The ovaries can go to sleep and wake
back up again. Ian Smith at the Royal
Marsden and I discussed this recently. He
went back and retrospectively reviewed
his institution’s experience with women
who had received chemotherapy, became
amenorrheic and were then placed on an
aromatase inhibitor.
About one quarter of those patients
had their ovarian function reemerge,
either by virtue of developing menses or
by having their estrogen levels increased.
Breast Cancer Update 2006 (7)
DR ROBERT W CARLSON: If I were to
treat 100 postmenopausal women with
endocrine therapy for early breast cancer,
the vast majority would walk out with a
prescription for an aromatase inhibitor
— usually anastrozole in my practice.
We have to establish a practice
pattern, and mine is to lead with an
aromatase inhibitor. It is interesting how
expert panels interpreted the emerging
aromatase inhibitor data differently.
Within 10 to 14 days of the initial
2001 ATAC presentation, the NCCN
panel had modified the guidelines to
allow anastrozole as an alternative to
tamoxifen as initial hormonal therapy
for postmenopausal patients with ER-positive
disease.
The ASCO panel initially believed
that tamoxifen should remain the
standard hormonal therapy, but that
guideline, over time, has also changed.
Currently, the NCCN and the ASCO
guidelines are essentially identical in
terms of up-front hormonal therapy.
The different methods of using
aromatase inhibitors or incorporating
them — initial aromatase inhibitor therapy
versus sequential after two to three
years of tamoxifen versus extended after
five years — have never truly been studied
in a randomized fashion, one against
another. The BIG 1-98 trial will give us
the first look at that sort of comparison.
The real question is whether tamoxifen
does something to prime the breast cancer
cells and cause the aromatase inhibitor
to be more effective in the switching
studies. Or, rather, is it that the population
of women and the characteristics of
their breast cancer change over time in
a way that would make the aromatase
inhibitors — or any hormonal therapy
— more effective?
I believe a substantial amount of data
exists to support the selection bias theory
that the population of breast cancer
patients over time is changing. You
would expect the endocrine-resistant,
receptor-positive breast cancer to recur
earlier, so those women are removed
from the denominator.
If you have a sensitive population and
an insensitive population with hormone
receptor-positive tumors — even with no
difference in efficacy between the hormonal
therapies — you should expect
to see an increasing effect the later in
time you initiate the therapy. However,
it’s hard to have a drug that’s so effective
down the road that you are able to regain
the loss of two to three absolute percentage
points that women may experience
when the drug is used in this context.
Breast Cancer Update 2006 (7)
DR VICTOR G VOGEL: How to approach
a patient who has received five years of
an adjuvant aromatase inhibitor is a challenging
question. Up until the 2005 San
Antonio meeting, I wasn’t certain what
the answer was to that question. But
I was heartened by the data that were
presented, both by Paul Goss and Jim
Ingle, on the continued follow-up of the MA17 trial patients and, particularly,
those patients who had initially been
assigned to placebo and then crossed
over to letrozole.
Two patterns were evident from those
data. The first was that the longer a
patient received the aromatase inhibitor
following five years of tamoxifen, the
greater the benefit. It is rare in medical
oncology to see a benefit that increases
as the duration of therapy increases. But
it was clear that the longer the duration
of therapy with letrozole was, the greater
the benefit was.
Comparing two years to four years,
the benefit almost doubled. So for our
patients at high risk, especially those with
larger tumors and those with positive
nodes, based on those data, we’re now
telling them they should continue to take
their aromatase inhibitor because we know
they’re at risk for a very long time — two
decades or longer — for recurrence, and
these data now show that longer therapy
may improve their outcomes.
The other question those data helped
us answer relates to patients who have a
gap between the end of their tamoxifen
therapy and the initiation of their
aromatase inhibitor therapy.
The patients who were initially
assigned to placebo after five years of
tamoxifen in the MA17 trial crossed
over to letrozole. Approximately 1,600
patients made the crossover, and their
average duration off therapy — that
is, the time between the end of their
tamoxifen and the initiation of their
letrozole — was about 30 months.
Even with that delay in the initiation
of the aromatase inhibitor, a statistically
significant benefit was demonstrated
with the so-called delayed initiation of
the aromatase inhibitor after tamoxifen.
Breast Cancer Update 2006 (5)
DR JULIE R GRALOW: The update of the
MA17 trial examined the patients who
originally received a placebo after five
years of tamoxifen as opposed to letrozole
and then at about 30 months, when
the study was unblinded, were offered
letrozole. Approximately two thirds of
those patients chose letrozole, and they
tended to be a higher-risk group.
Those patients had an average gap of
30 months without any endocrine therapy.
Despite that and the fact that they
were a good eight years out from their
diagnosis, a reduction appeared across
the board in every type of breast cancer
recurrence — contralateral, in-breast
and distant. It’s impressive.
We saw the updated analysis for the
MA17 trial at the San Antonio meeting
in 2005, and at that point I began to at
least offer patients the option of going
back on an endocrine agent if they’d
been off everything for a couple of years,
especially if they were at high risk.
Although it might offer some benefit
10 years later, the duration off therapy
in the MA17 trial was approximately 30
months, so I consider restarting endocrine
therapy for patients up to three
years off treatment. That’s arbitrary, but
you have to pick some time period.
Cancer Conference Update, San Antonio
Breast Cancer Symposium 2005
DR PETER M RAVDIN: The problem
with the extended letrozole trial (NCIC-CTG-MA17) was that the patients were
unblinded at 2.4 years, and because most
patients then switched over to the active
agent, we will never know with any
certainty what would have happened had
they been unblinded at five years. That
is a shame because we are going to be
treating these patients for five years, so it
would have been nice to know the differences
in toxicity and efficacy between
the two arms. The data for one or two
years are complete because most of the
patients had gone through those years.
There were a lot of data in year three, a
modest amount in year four and almost
no data for the fifth year.
An analysis of relapse risk within
each year could then be performed. This
was possible not only for years one and
two but also for year three, when it
seems that the relative benefit was greater,
which is interesting and reassuring.
That was also the case in year four. That
analysis used year-by-year hazards to
determine whether benefit was attenuating,
staying as strong or becoming stronger.
Although we will never know what
it would have been if the trial had been
unblinded at five years, we are somewhat
reassured by the results of this analysis
that going beyond 2.4 years of treatment
is reasonable.
Breast Cancer Update 2006 (8)
DR HARRY D BEAR: In my practice, by
and large, the postmenopausal patients
who do not have osteoporosis are receiving
aromatase inhibitors up front. The
ATAC results are difficult to dispute.
For patients who have been on tamoxifen
for a year, I haven’t jumped to switch
them to an aromatase inhibitor.
I will probably follow the paradigm of
some of the other trials and leave them
on tamoxifen for a couple or three years.
Then I’ll switch them over. I believe they
will obtain some bone-density benefit by
staying on tamoxifen for a while and start
out at a better baseline when we switch
them over to an aromatase inhibitor.
NSABP-B-42 will address the question
of duration of therapy. It will look
at the group of patients who have been
on five years of either a combination of
tamoxifen and an aromatase inhibitor or
an aromatase inhibitor alone. The trial
will determine whether those patients
should receive an aromatase inhibitor
for another five years. It’s a five- versus
10-year question, reminiscent of the
NSABP-B-14 rerandomization.
Interview, September 2006
DR NORMAN WOLMARK: The NSABPB-42 trial just opened. It has a sample
size of about 3,800, and of course one of
the questions that remains unanswered
is the duration of an aromatase inhibitor.
We went through this process and it took us years to determine the optimum
duration of tamoxifen therapy, and at
the end of the day there was enormous
surprise from the B-14 data that not only
is 10 years not as good as five, but it is
also somewhat detrimental. We believe
it’s important to address the duration of
an aromatase inhibitor, and this is what
NSABP protocol B-42 will be doing.
The data with aromatase inhibitors
from the multiple trials have all been
positive. The duration question remains
relatively unaddressed.
We have seen trials that have introduced
aromatase inhibitors after a period
of tamoxifen and have shown an
advantage. We’ve seen direct head-on
comparisons between aromatase inhibitors
and tamoxifen up front also showing
an advantage, and we’re waiting to
see the results of a trial that starts with
an aromatase inhibitor and sequences it
with tamoxifen.
NSABP-B-42 Protocol July 2006; nsabp.pitt.edu.
In the adjuvant setting, AIs have demonstrated
activity in three distinct clinical
situations. In the first situation,
an AI was compared to tamoxifen as
initial adjuvant hormonal therapy in
patients with resected operable breast
cancer. The ATAC trial demonstrated
that 5 years of anastrozole significantly
improved disease-free survival (DFS)
when compared to 5 years of tamoxifen.
More recently, the BIG 1-98 trial also
demonstrated improved DFS as well as
distant DFS for 5 years of letrozole
compared to 5 years of tamoxifen.
In the second situation, an AI was
compared to tamoxifen in patients who
had already received 2-3 years of adjuvant
tamoxifen. In three randomized trials
(the IES trial [International Exemestane
Study], the ABCSG-8/ARNO 95 trial,
and the ITA trial [Italian Tamoxifen vs
Anastrozole]), 2-3 years of an AI (exemestane
or anastrozole) improved disease-free
survival compared to 2-3 years of
tamoxifen in patients who had already
completed 2-3 years of tamoxifen therapy.
In the third clinical situation, an AI
was evaluated as extended adjuvant hormonal
therapy following completion
of 5 years of adjuvant tamoxifen. The
NCIC-MA17 trial compared 5 years
of letrozole with 5 years of placebo in
patients who had already completed 5
years of adjuvant tamoxifen and demonstrated
significant improvement in disease-free survival in favor of the group
that received the AI.
Based on the results from these trials,
AIs are increasingly utilized as adjuvant
therapy in these three clinical situations.
At this time, there are no available
results from trials that directly compare
these different approaches for using AIs.
Thus, the best setting for the adjuvant use of AIs cannot be readily determined
at present.
Breast Cancer Update 2006 (1)
DR PAUL E GOSS: We don’t know what
the appropriate approach is to selecting
one of the three aromatase inhibitors
in the up-front setting. I have the good
fortune of chairing a key study in this
regard. The MA27 study will complete
accrual in 2006, and it is addressing
precisely that question of whether there
is an optimal aromatase inhibitor. The
randomization is between the steroidal
exemestane and the nonsteroidal
anastrozole.
In the meantime, there are ample data
to say these compounds are different in
terms of their biochemical and preclinical
effects. But in the clinic, with the
present data, there is no evidence of a
wide difference between these drugs.
So I think that one has to restrict one’s
choices to the approved therapies by the
regulatory agencies and the published
evidence-based data.
John W Berry. Are all aromatase inhibitors
the same? A review of controlled
clinical trials in breast cancer. Clin Ther 2005;27(11):1671-84.
There may be important clinical differences
between the AIs. However, data
from direct comparative clinical trials
are limited, and making comparisons
across trials is difficult given differences
in design, methodology, patients,
and endpoints. At the present time, the
choice of an AI for clinical use should
be based on the strength of the data
within the distinct clinical scenarios:
neoadjuvant therapy, adjuvant therapy,
or advanced/metastatic disease.
Breast Cancer Update 2006 (1)
DR AMAN U BUZDAR: As the safety
data for the three aromatase inhibitors
are emerging, we see that they are
quite different. In the package insert
for exemestane, a small but definite
increased risk of cardiac dysfunction
is noted. If you consider the letrozole
data from the BIG trial, at 25 months
a small but definite increased risk of
cerebrovascular accident and myocardial
infarct is evident. However, in the
68-month follow-up data for the ATAC
trial, we see none of those risks with
anastrozole. If you examine the cardiac
deaths, it is 49 with anastrozole versus
46 with tamoxifen, and cerebrovascular
accidents are substantially reduced with
anastrozole compared to tamoxifen.
An interesting study presented at
the 2005 San Antonio Breast Cancer
Symposium evaluated 90 healthy, postmenopausal
volunteers who received,
in a blinded fashion, up to 24 weeks
of anastrozole, letrozole or exemestane.
When the effects on the lipids were
examined, they were found to be totally
different. We have to be aware of the
different effects and realize that not all
aromatase inhibitors are alike and that it
does matter which one we select.
Jean Marc Nabholtz, Joseph Gligorov. Cardiovascular safety profiles of aromatase
inhibitors: A comparative review. Drug Saf 2006;29(9):785-801.
A significantly reduced risk of thromboembolic disease was observed for all
three AIs compared with tamoxifen.
Anastrozole is, at this point, the only
AI with a detailed benefit-risk profile
from over 5 years’ follow-up in the adjuvant
setting.
Thus far, no apparent CV-safety
concerns have emerged. Preliminary data
on letrozole and exemestane suggest that
longer follow-up is needed for these two
AIs before being able to fully assess their
respective long-term CV toxicity profile.
The present differences in CV-safety
profiles suggest that third-generation
AIs should not be considered as equivalents
in clinical practice.
Meet The Professors 2006 (3)
DR DEBU TRIPATHY: As time goes on,
less and less of a distinction can be made
between the aromatase inhibitors. Up
front, I don’t have a strong preference.
We certainly have data for anastrozole
and letrozole. I tend to use anastrozole
simply because it has longer safety data.
There we have the largest number of
patients that have been followed, so in
my mind, there’s more confidence in the
safety profile.
BONE AND AROMATASE INHIBITORS
Breast Cancer Update 2006 (5)
DR GRALOW: The five-year bone density
substudy of the ATAC trial was very
interesting. The fracture rates on that
trial were approximately 11 percent in the
anastrozole arm and about 7.5 percent
in the tamoxifen arm at 68 months of
follow-up.
However, we were trying to determine
who should receive bisphosphonates up
front and how often we should follow
bone density studies. I believe the
ATAC data that Rob Coleman presented
at ASCO showed that not everyone
needs a DEXA scan every year or a
bisphosphonate up front.
What was surprising to me but very
reassuring was that none of the patients
who started the ATAC trial with a normal
bone mineral density — a T-score
better than minus one — were osteoporotic
after five years of treatment,
although approximately 50 percent had
become osteopenic.
We expect about a two to three percent
bone loss during the five years simply
based on aging, but in the tamoxifen
arm, approximately 15 to 20 percent
of the patients went from normal to
osteopenic, and the rate was 50 percent
for patients who received anastrozole.
Aging happens even to the best of us,
but I believe these data show us that if
the patient started with a normal bone
mineral density, her chance of becoming
osteoporotic after five years as a result of
receiving an aromatase inhibitor in that
study was zero.
ADHERENCE TO LONG-TERM ORAL
ENDOCRINE THERAPY
Interview, August 2006
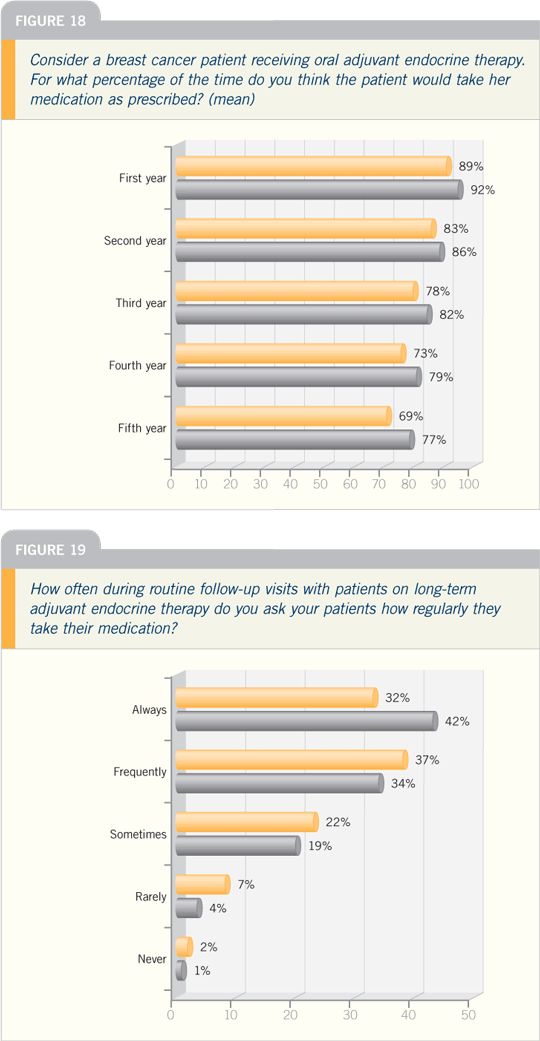
DR VICTOR VOGEL: In terms of patients
stopping long-term medications,
published data show that the decay over
time is very high.
There are more data published on
tamoxifen than on the aromatase inhibitors,
but even some aromatase inhibitor
data show that as many as one third of
patients stop their medication within the
first year and by the second year, as many
as half of patients have stopped taking
their aromatase inhibitor. This should be
distressing to all of us.
A number of barriers exist, such as
cost and side effects. An equally important
barrier is patients’ misperception
that if time has transpired and they’re
doing well — they’re coming back for
their second and third annual visits and
their mammograms are fine, their physical
exams are normal and they’re asymptomatic
— that their risk has passed and,
therefore, it’s not necessary to continue
the medications.
It’s important for oncologists to be
aware that patients are stopping their
medication and that we need to regularly
ask patients whether they’re taking the
medications daily and determine whether
there are any barriers to doing so — be
it cost or symptoms or perceptions about
the risk of recurrence.
I don’t think it was well recognized in
our treatment community that patients
were stopping their medications. We are
not in tune with the reality, and when
you actually examine prescription refills
and availability of medications over time,
in fact, patients are not being compliant.
I think the first step is for us to recognize
that patients aren’t compliant and to
stop pretending that they simply follow
our directions because we told them this
is what they need to do.
We need to ascertain if the patient is
compliant, and there are many strategies
we can use to do this, be it pill counts,
pharmacy records or simply asking
patients. We need to constantly ensure
that what we believe the patient is doing
is what they’re actually doing. The data
would suggest that, in fact, patients are
not following our advice.
Interview, June 2006
DR D LAWRENCE WICKERHAM: We
spend a fair amount of time and energy
educating our physicians, nurses and
coordinators about the importance of
compliance and adherence. Within the
context of a clinical trial, you can pick
your patients a little, so we try to identify
those individuals most likely to
be compliant with the regimen — not
only taking their pills but also receiving
their follow-up exams, mammograms
and so forth. Then we institute a number of strategies to help maintain
that level of compliance during the
course of the trial.
We design our trials with a built-in
level of noncompliance. Clearly, you
want patients to take their medications
so they can obtain the maximum benefit
and so that the study results, both
benefit and toxicity data, are as accurate
as possible.
Applying that information to the
general population of patients who are
not in clinical trials has recently become
an area of interest in both the treatment
and the prevention settings. As
we have more oral agents in oncology, it
becomes increasingly important for us
to be thinking about how to keep our
patients on these therapies.
The most important thing is to ask
the patient in an open fashion whether
they’re having any difficulties taking
their medication. Without making it
sound threatening, that should be asked
at each follow-up visit, and the importance
of taking their medication as prescribed
should be reinforced. Patients
should be told to announce any difficulties
in taking their medications, be
it side effects, toxicities or economic issues. These can all be addressed, but
only if they are described.
Interview, June 2006
DR ROWAN T CHLEBOWSKI: Adherence
to oral hormonal therapy has received
minimal attention, but it’s an area of
increasing interest.
I believe that because chemotherapy
is perceived as being a burden and difficult,
the concept is that when you are
done with the chemotherapy, you are
done with the heavy lifting. Indeed,
some practices provide diplomas, like
graduation, after chemotherapy.
When oncologists see patients three
and four years out in a 12-minute slot,
when we reassure them that they are
doing fine and give them a six-month prescription
for aromatase inhibitors, it’s easy
to understand how patients might perceive
that they are done with their cancer.
If you’re taking pills, you’re admitting
that you have a problem. And if
you can stop the pills, mentally, in a certain
sense, you are putting the problem
behind you. When women get three or
four years out after a breast cancer diagnosis,
they’d like to think the problem is
behind them.
The other issue is cost, and when
a woman says, “That’s too expensive,”
I ask her, “Do you have the money to
pay for the medication?” Some women
don’t, but many women, in effect, are
spending the money on something else.
Then I’ll ask them, “If you don’t want
to spend the money on the aromatase
inhibitor, what do you plan to spend the
money on?”
That lets them know that I think it’s
important that they’re making this choice.
And then I remind them that this is quite
different than considering a decision to
get dial-up or cable internet, where, at the
end of two years, you have saved $2,000,
and you were willing to put up with the
slowness of the internet speed.
Here, you’re doing something more
than that. You’re making a bet. Because
I tell the women that there’s appreciable
cost to themselves and their family for a
breast cancer recurrence. If the patient
develops a recurrence, that could cost
thousands and thousands of dollars and
jeopardize their entire financial bearing.
You have to judge very carefully a decision
that may effect the risk of a recurrence.
Breast Cancer Update 2004 (7); 2006 (3)
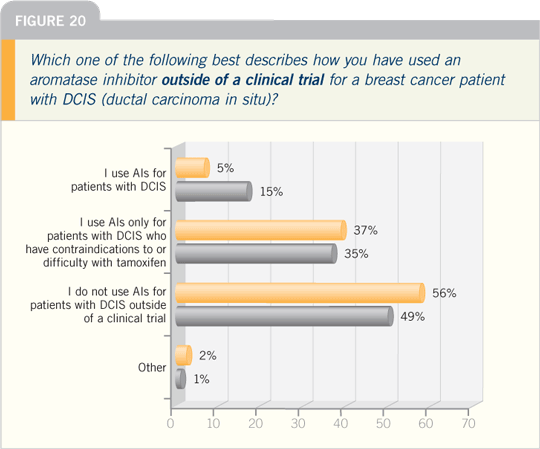
DR PATRICK I BORGEN: NSABP-B-35
and IBIS-II are important trials, both
comparing anastrozole and tamoxifen
in postmenopausal patients with DCIS.
Aromatase inhibitors have already proved
to have a significant effect in invasive
cancer, and it’s highly likely they will
affect DCIS as well.
We know that the majority of DCIS
lesions are likely to be ER-positive. Craig
Allred has shown that age per age, tumor
for tumor, DCIS is even more likely to
be ER-positive than invasive cancer. If
that’s true, then we have even more reason
to be optimistic about the studies of
aromatase inhibitors in DCIS.
We have viewed tamoxifen as a
highly appropriate option for treating
a patient with ER-positive DCIS since
the NSABP-B-24 trial. However, when
we consider risks, benefits and quality-of-life issues, it’s common for our New
York patients to demur, so we probably
have one of the lowest percentages of patients with ER-positive DCIS on
tamoxifen in the country.
The same can be seen in our prevention
setting, in which we’ve not been
successful in getting patients to take
tamoxifen.
The two most obvious concerns about
tamoxifen in these settings are endometrial
cancer and gynecological events.
Even when we provide the raw numbers
on how infrequent those events are,
because we are talking about minimal,
if any, impact on long-term survivorship
and moderate impact on local control, it
simply is not an attractive option.
We’d like more information about
DCIS and aromatase inhibitors, but
since the initial publication of the ATAC
data, aromatase inhibitors have become
our endocrine therapy of choice for postmenopausal
patients with ER-positive,
invasive cancers. That literally happened
overnight, like gangbusters, and so a
“bleed over” to postmenopausal patients
with DCIS is natural.
In my clinical practice, it’s clear that
the aromatase inhibitors are vastly better
tolerated than tamoxifen in postmenopausal
patients.
Our surgeons are beginning to give
first-line endocrine therapy without a
mandatory consult from medical oncology.
We perform bone density tests before
we start our patients on aromatase inhibitors,
and treating these patients has
been satisfying.
Select publications
|
