|

Breast Cancer Update 2006 (5)
DR HAYES: For older women, I believe
the jury is out regarding the potential
benefits of chemotherapy. The issue has
two components. One is whether — for
some mysterious reason — chemotherapy
doesn’t work as well in older women
as in younger women. The second is
whether the toxicities are greater for
older women and, therefore, the benefit-to-toxicity ratio is smaller.
Another component is whether
the number of life-years saved will be
lower for older women and therefore
not acceptable. An 80-year old woman
on average has another 10 years to live,
but the number of life-years saved for
her will be lower than for a 50-year-old
woman for the same potential reduction
in recurrences. Peter Ravdin has begun
to build that into Adjuvant! Online.
It’s not something we normally talk to
patients about, but I believe it is part of
the equation.
The CALGB-49907 study, which is
restricted to patients over age 65, assumes
that chemotherapy is beneficial. It is not
a trial of chemotherapy versus none. The
question is whether in this older age
group one type of chemotherapy might
be more acceptable by being less toxic.
Patients either receive one of the standard
regimens — AC or CMF — or
capecitabine. A critical part of the study
is to determine whether capecitabine is a
more acceptable regimen.
Breast Cancer Update — Think Tank
Issue 2, 2006
DR HOPE S RUGO: I would consider
adjuvant chemotherapy for an otherwise
healthy woman in her eighties with
triple-negative disease, but even more
so for the patient with an ER-negative,
PR-negative and HER2-positive tumor,
for whom we know that recurrence is
heavily weighted in the first two or three years. As the survival of our population
increases, these 81- and 82-year-old
women who don’t have major medical
problems are reasonable candidates for
limited approaches to chemotherapy.
This must be within the limits that
we all know to be important, such as
understanding morbidities. That’s one of
the reasons Adjuvant! Online can be very
useful in directing physicians who are
treating older patients. First, in this older
population, the patients with hormone
receptor-negative disease are the ones for
whom we are going to be thinking about
chemotherapy.
Then, in regard to morbidity, if a
patient has a major morbidity, such as
heart failure, and is not going to be alive
in three years, that is not the patient we
should be treating with chemotherapy.
Breast Cancer Update — Think Tank
Issue 2, 2006
DR CLIFFORD HUDIS: In the quantifiable,
objective ways in which we assess
toxicity, you cannot support the argument
that dose-dense therapy is more
toxic. In CALGB-9741, it appears to be
equivalent or perhaps less toxic in many
ways. The one toxicity that stood out in
the original Citron paper was the high
rate of packed red blood cell transfusions,
which appears to be abrogated
with the use of erythropoietin or darbepoetin
as prophylaxis. It’s my subjective
opinion that patients stay on schedule
more easily when they receive every two-week
therapy with growth factor support
than when they are treated with an every
three-week schedule. When patients
can’t plan their therapy, it is an annoyance,
and it can reduce quality of life.
Also, completing therapy faster is
always worthwhile. We’ve taken the
position that unless we have a compelling
reason not to administer a growth factor,
we use every two-week therapy for everybody
who receives AC and a taxane.
Breast Cancer Update 2006 (7)
DR CARLSON: Docetaxel administered
every three weeks at 100 mg/m2 is a
reasonable taxane to use following AC
chemotherapy. I have no difficulty with that. ECOG trial E1199 suggested equal
efficacy to paclitaxel in that setting.
Perhaps a little more toxicity, especially
febrile neutropenia, occurred with the
every three-week regimen. Given the
increased frequency of febrile neutropenia,
growth factors would be reasonable
to use with that dose and schedule.
TAC certainly causes febrile neutropenia
with a high enough frequency
that growth factors should be used.
The NCCN Breast Cancer Treatment
Guideline specifies the use of growth factors
with two of the adjuvant chemotherapy
regimens. One is TAC and the other
is a dose-dense chemotherapy regimen.
Breast Cancer Update 2006 (6)
DR I CRAIG HENDERSON: I see dose-dense
AC without paclitaxel being administered
off protocol in my own clinic. I
started a couple of patients in the last few
weeks on dose-dense adjuvant chemotherapy
and discussed it with some of my
colleagues, and in fact, they are doing this
in the university setting. In CALGB-9741,
which compared sequential doxorubicin,
paclitaxel and cyclophosphamide versus
concurrent AC followed by paclitaxel at
14- and 21-day intervals, we can’t separate
which is the critical factor — the AC or
the taxane. We will have to wait and see
what the science says.
Breast Cancer Update 2006 (7)
DR CARLSON: I believe that every two-week
AC without a taxane with only
growth factor support is a reasonable
regimen, and I use it for the patients for
whom I do not consider a taxane necessary.
It’s based on the belief — and it’s
just a belief, it’s not yet proven — that if
dose-dense AC followed by paclitaxel, or
the ATC dose-dense regimen, is superior,
it’s likely that every two-week AC
should be superior or at least equal to
every three-week AC.
I’m impressed at how nontoxic this
regimen is when you use growth factors.
I believe women like to get through
these therapies quickly, and you shorten
the duration of treatment with the dose-dense
regimens.
Breast Cancer Update 2006 (7)
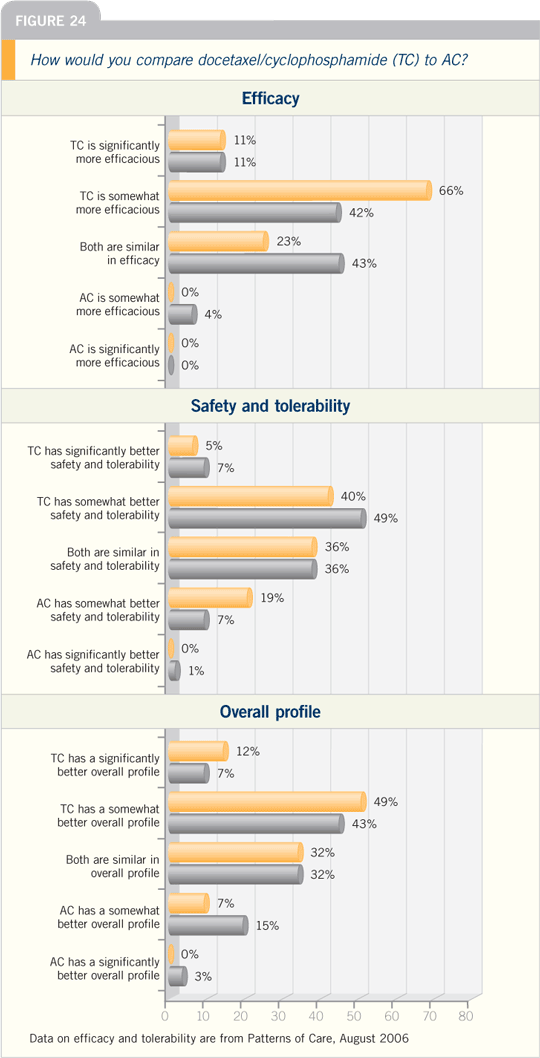
DR MARK D PEGRAM: The presentation
by Steve Jones at San Antonio
2005 of the US Oncology adjuvant trial
of docetaxel/cyclophosphamide versus
AC was an exciting presentation, and I’m not surprised at all by the data.
Steve presented a randomized trial for
patients with early-stage breast cancer,
approximately 40 to 50 percent of whom
had node-negative disease. They were
randomly assigned to four cycles of AC
versus four cycles of TC.
They showed a significant relapse-free
survival advantage with the TC
compared to the AC arm, and a numeric
trend even appeared in the survival analysis,
although it hasn’t reached statistical
significance yet. Steve Jones concluded
— and probably rightly so — that this
constitutes a new regimen that replaces
AC. If you were going to use a four-cycle
regimen, you probably wouldn’t want to
use AC anymore, based on this data set.
I was also favorably surprised by the
toxicity and safety data. The TC was
well tolerated compared to AC. It goes
to show that we probably underestimate
the toxicity of AC routinely because
we’re so used to prescribing it.
I saw a young woman recently in my
clinic with newly diagnosed doxorubicin
cardiotoxicity after adjuvant therapy for
what will probably be curable breast cancer.
It’s sobering and scary when you see
cases like this.
Breast Cancer Update 2006 (6)
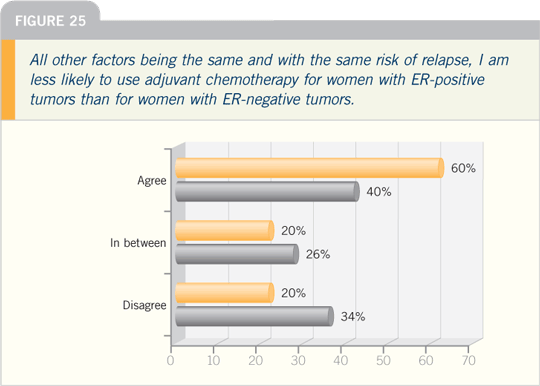
DR MARC E LIPPMAN: Almost 30 years
ago, we published, in The New England
Journal of Medicine, that patients with
ER-negative disease responded more
frequently to chemotherapy than patients
with ER-positive disease.
Those data have been replicated in
the meta-analyses conducted in England
by Sir Richard Peto and his collaborators. The clue as to why that occurs is
obtained if you observe recurrence rates
for women with breast cancer as a function
of whether their disease is ER-positive
or ER-negative. It is commonly said,
but that doesn’t necessarily make it the
truth, that having ER-positive disease is
a good prognostic factor. The data show
— and this has now been shown several
times — that early on, if your disease is
ER-positive, your relapse rates are lower.
Over time, the patients with ER-negative
disease, who relapse at a higher rate,
initially stop relapsing, perhaps because
most of the ones with bad prognoses have
already died, whereas the patients with
ER-positive disease continue to relapse,
and those lines actually cross. At about
10 to 15 years, you’re worse off having
ER-positive than ER-negative disease.
Breast Cancer Update 2006 (7)
DR CARLSON: The analyses of dose-dense
chemotherapy and TAC in
hormone receptor-positive patients are
provocative. Dose-dense chemotherapy
showed very little benefit in receptor-positive
breast cancer, whereas not much
difference in efficacy appeared between
the patients with ER-negative and ER-positive
disease in the TAC study. Those
are indirect comparisons, so I’m not sure
we can make much of that specific finding.
It’ll be interesting to see, as ECOG-E1199
unfolds, if a differential responsiveness appears with docetaxel versus
paclitaxel based on ER status, because
that’s what you’d have to hypothesize.
Breast Cancer Update 2006 (7)
DR PEGRAM: Determining a chemotherapy
regimen for patients with ER-positive
disease depends on their age, et cetera. If
they’re getting on in years, I’m more likely
to use AC followed by weekly paclitaxel,
for example, because that’s so well tolerated.
If they are young, fit, in their thirties,
have no comorbid medical illnesses and
have a number of positive nodes, I would
have no hesitation using TAC because we
participated in some of those TAC trials
and we’re comfortable with the regimen
when we use pegfilgrastim.
Breast Cancer Update 2006 (8)
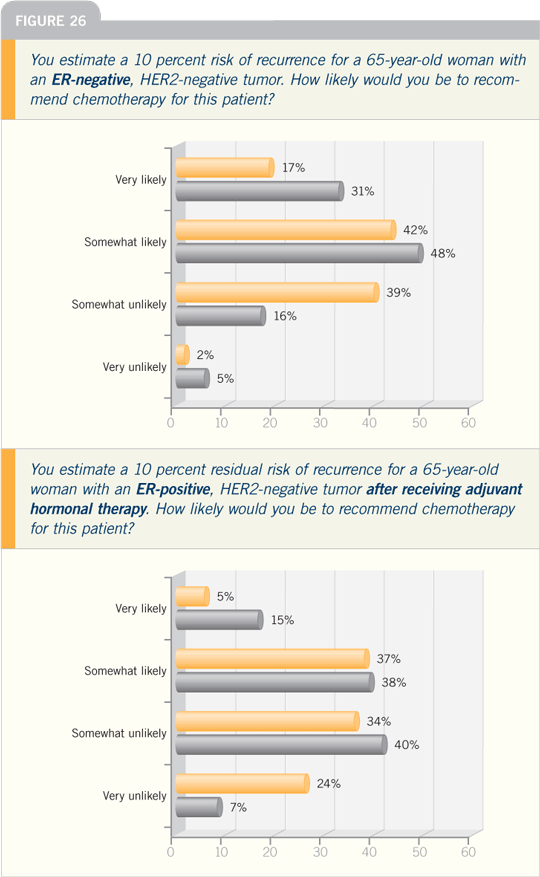
DR RAVDIN: In the most recent Oxford
Overview chemotherapy data, the correlation
between estrogen receptor status
and impact on outcome was hotly debated
and complicated by the fact that age
has to be taken into account in evaluating
the first-generation trials. Overall,
it looks as if ER status did not make a
difference.
In contrast, ER status appears to
make a difference in older patients.
Patients with ER-positive tumors benefited,
although the benefit was smaller
than in those with ER-negative disease
— approximately a 2:1 difference. So
ER status is important in therapy, but
its importance is more obvious among
older patients.
Breast Cancer Update 2006 (7)
DR VOGEL: Using archival tissue blocks
from past trials, Genomic Health and Dr
Soon Paik from the NSABP analyzed
about 200 genes that were reported to
possibly relate to outcome in breast cancer.
They narrowed that set down to just 16
genes that could be sorted into logical
groups based on the estrogen receptor, the
HER2 protein and proliferation and invasion
characteristics of the cells.
That set of 16 genes plus five reference
genes were used to see if breast cancer
patients could be sorted into prognostic
and predictive groups. When I say “prognostic” I mean to predict the
likelihood of recurrence, and when I say
“predictive” I mean to predict patients
who would benefit from chemotherapy.
So the investigators examined the archival
subsets and were able to determine
that those 16 genes and five reference
genes could be used to sort patients along
a continuum they called the recurrence
score, which varies from zero to 100.
Using simple mathematic regression procedures,
that recurrence score could then
be translated into a probability of recurrence
over 10 years.
The investigators were also able to
determine that patients who had low
recurrence scores — that is, scores lower
than 18 — benefited from hormonal
therapy but derived no additional benefit
from the addition of chemotherapy to
their hormonal therapy regimens.
Conversely, patients with high recurrence
scores — scores of 31 or higher
— showed a clear, statistically significant
and large benefit when cytotoxic chemotherapy
was added to hormonal therapy
— that is, tamoxifen. In the intermediate
group, the group with scores between 18
and 30, no benefit was apparent from the
addition of chemotherapy, but the confidence
intervals — the statistical certainty
of no benefit — were not established.
What came out of that work was
the Oncotype DX assay from Genomic
Health. It is commercially available and
essentially allows selection of patients
for hormonal therapy alone or hormonal
therapy with chemotherapy in the high-risk
group.
In the intermediate-risk group, we’re
left with some uncertainty. An Intergroup
clinical trial, known as the TAILORx
study, is for patients with ER-positive,
node-negative, early-stage — Stage I,
small Stage II — breast cancer. Patients
with intermediate recurrence scores will
be randomly assigned to chemotherapy
or no chemotherapy, in addition to their
hormonal therapy.
Breast Cancer Update 2006 (6)
DR C KENT OSBORNE: I believe the
Oncotype DX is well done — well standardized
and well validated. It produces good results. For laboratories that don’t
perform a high volume of assays, where
estrogen receptor and HER2 assays are
not reliable, the Oncotype DX would
provide a much more reliable estrogen
receptor test, because the estrogen receptor
is such an important part of the
generating signal.
So for institutions that don’t measure
these things very well, I believe they
should use Oncotype DX. In terms of
trying to decide who has a worse prognosis
and who might need to have adjuvant
chemotherapy for a small, node-negative
tumor, I believe the Oncotype DX can
be helpful.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR HAYES: The reason we are conducting
the TAILORx trial is that we are in
enormous equipoise about the addition
of chemotherapy for the Oncotype DX
intermediate recurrence score group. I
believe we all agree that the addition of
chemotherapy for the low recurrence score
group is below our radar screen in terms
of benefit, and most of us also agree that
patients with high recurrence scores have
at least a five to six percent or higher absolute
reduction in recurrence rates. Those
are the patients for whom we would probably
recommend chemotherapy.
But for the intermediate group, whether
we define it by a recurrence score of 11
or 18, we are in great equipoise. That
is especially true because the aromatase
inhibitors may be more effective than
tamoxifen so patients have a better prognosis
than the patients in the NSABP
study. I also believe that doxorubicin
and the taxanes will be more effective
in patients with lower ER and higher
HER2 levels.
So depending on where you are in that
intermediate group, you may have a better
prognosis than we think you have, but you
may have a higher proportional reduction
than that achieved with CMF. The randomized
portion of that trial is critical.
Interview, September 2006
DR WOLMARK: The TAILORx trial is
following up on the findings of the value of the Oncotype DX assay in assessing
the risk of recurrence and predicting the
benefit from chemotherapy. It’s an interesting
and ambitious trial that is scientifically
compelling and that we would
like to see completed.
Select publications
|
