| |
Editor’s Note: The new toxicology of solid tumor oncology |
This issue of Patterns of Care continues our efforts
to quantitatively assess clinical practice patterns in a
number of different areas of oncology. For this unique
foray — our first ever into supportive care — we commissioned
a web-based survey of 100 US-based medical oncologists with
the goal of better understanding how these individuals manage
the side effects and complication risks associated with many
common systemic agents used to treat solid tumors, particularly
and specifically, “targeted biologic agents.”
The snapshot that emerges suggests that the recent introduction
of a litany of new therapies and regimens has forced
oncologists to suddenly wear a number of new primary care hats
in order to manage a spectrum of novel toxicities. Below find a
few thoughts on the challenges associated with administering a
number of these agents and combinations that were not widely
used prior to the year 2000, along with some related “stat bites”
from the survey.
— Neil Love, MD
DrNeilLove@ResearchToPractice.com |
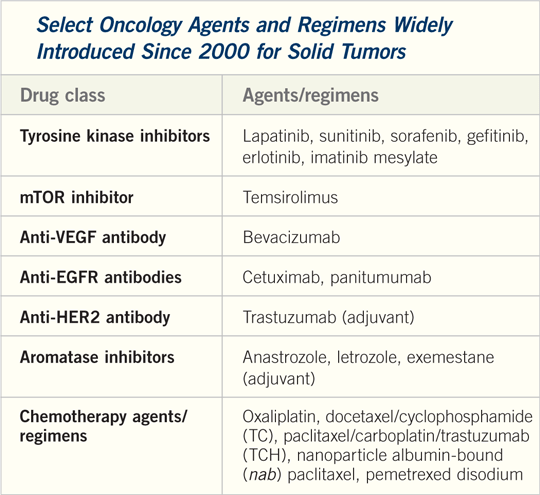 |
1. The many and sometimes not so purely
targeted TKIs
| 91% |
Percent of oncologists who have prescribed sunitinib or sorafenib (Figure 30). |
| 50% |
Median estimate by oncologists of the percent
of patients experiencing dermatologic toxicity
while receiving erlotinib (Figure 19). |
The good news is that many of these oral agents are associated
with impressive efficacy, and the associated risks are rarely life
threatening. The less-than-good news is that it takes meticulous
attention and constant vigilance to keep patients on these
therapies because of a number of side effects, including fatigue,
hand-foot syndrome and diarrhea. It will be fascinating to
see how these challenging toxicities impact the current adjuvant
renal cell cancer trial comparing one year of sunitinib to
sorafenib to placebo in a double-blind design. Investigators tell
us that it’s pretty clear who is receiving an active drug, and many
patients have difficulties reaching the one-year point.
Despite these challenges, the TKIs are having a significant
impact in a number of interesting tumor types. In hepatocellular
cancer, after more than 100 Phase III randomized trials of
systemic therapy failed to change the outcome of these patients,
for the first time sorafenib has been demonstrated to improve
survival, and this agent is now the standard first-line systemic
therapy. It is now being investigated in combination with local
treatments such as RFA (radiofrequency ablation) or TACE
(transarterial chemoembolization).
Similarly, clinical investigators specializing in renal cell cancer
tell me their waiting rooms are now much more crowded, in part because patients are living longer as a result of the impact
of sunitinib as the standard first-line therapy for metastatic
disease. Waterfall plots are equally impressive, demonstrating
benefits for most patients.
In breast cancer, lapatinib is a welcome new alternative for
patients with HER2-positive disease, although recent reports
of significant diarrhea and skin rash when combined with
paclitaxel have led to modifications of the designs of a number
of new adjuvant and neoadjuvant trials.
Perhaps the most exciting TKI story (in solid tumors anyhow)
is in non-small cell lung cancer, in which approximately
10 percent of patients — mostly nonsmokers — have EGFR
tumor mutations that predict exquisite sensitivity to erlotinib
or gefitinib. While these agents also often bring with them a
troublesome rash and even a strange abnormal eyelash growth,
the response in these patients is about as close as we’ve come in
solid tumors to the magic that is imatinib in CML.
2. Bevacizumab
| 23% |
Percent of oncologists who consider primary
tumor location of a non-small cell lung cancer
to be the most important risk factor for bevacizumab-associated hemoptysis (Figure 34). |
Bevacizumab — a highly interesting anti-VEGF antibody — has
been a major topic of discussion since Herb Hurwitz’s stunning
ASCO 2003 presentation demonstrating a progression-free and
overall survival advantage to adding this agent to chemotherapy
(IFL) in metastatic colon cancer.
Bev is now out there in breast, lung and colon cancer and a
bunch of other less common tumors, but I still don’t hear anyone — not even Lee Ellis or Rakesh Jain — explaining for sure
how this agent works. We also have yet to find an effective predictor
of response or toxicity, although emerging evidence about
hypertension and SNPs — as discussed in a fascinating paper
by Schneider et al in the October 1st issue of JCO — are at least
providing some hints.
One of the most important qualities of bev is that it doesn’t
seem to make many patients feel more ill, and while hypertension
and proteinuria are not infrequent, these problems
are reported to be relatively easy to control for most patients.
Serious complications with bev are uncommon, and the modest
increase in arteriovenous events associated with the agent needs
a lot more definition.
The scariest acute bev toxicity is the pulmonary hemorrhage
seen in lung cancer. This event — which may be part of a
brisk tumor response — is, thankfully, quite infrequent (one to
four percent) and may be less of a concern in a clinical scenario
(metastatic non-small cell) in which more than 80 percent of
patients will die within two years despite “standard treatment.”
It’s interesting that a quarter of docs believe that central tumor
location is the most important predictor of this potentially catastrophic
event, although Alan Sandler, the principal investigator
of ECOG trial E4599, the seminal bev study in metastatic
lung cancer, repeatedly has rejected this association.
The toxicities of this agent will be totally reexamined if it
works in the adjuvant setting, a question being addressed in
breast and lung cancer, but most critically, in the colossally
important NSABP-C-08 trial and the AVANT study, arguably
the most important current oncology trials currently complete
and waiting for results.
3. Cetuximab/panitumumab
| 56 |
Mean number of patients with colorectal cancer
treated with cetuximab in the past year by
clinical investigators in GI cancer (For oncologists
in practice, this number was 16.) |
| 80% |
Median estimate by oncologists of the percent
of patients experiencing dermatologic toxicity
while receiving erlotinib (Figure 19). |
As of ASCO 2008, this class of agents is now a consideration for
the most common solid tumor, as discussed on the Lung Cancer
Update audio series by the principal investigator of the FLEX
trial, Dr Robert Pirker, and the ASCO discussant of this historic
study, Dr Tom Lynch. As noted in the above stat bite, oncologists
in community practice know a lot about cetuximab and
its cousin, panitumumab, from treating colorectal cancer, and a
major quality of life concern is dermatologic toxicity.
All docs in practice are eager for effective solutions to this
visible and disturbing problem. One strategy that would certainly
help alleviate this problem would be to clone Dr Mario
Lacouture, a dermatologist at Northwestern University who focuses his entire practice and clinical research on EGFR-related
dermatologic side effects. Mario treats this dilemma
as both an art and a science, and perhaps as an alternative to
genetic engineering, we can help him encourage and train other
dermatologists to give up a few cosmetic procedures and develop
expertise in this area.
4. mTOR inhibitor (temsirolimus......TEM-sir-OHli-mus)
| 11% |
Percent of oncologists who can correctly pronounce
temsirolimus (kidding) |
It took me a while to get the hang of pronouncing the name of this
recently introduced agent, but finally, just like bevacizumab and
trastuzumab before it, temsirolimus started flowing out naturally.
Most docs in practice have only used temsirolimus (got the
hang of it yet?) a couple of times, considering that it is both new
and so far confined only to advanced renal cell cancer.
However, as time passes and this interesting agent enters
into other treatment areas, along with brethren like everolimus
(I actually prefer the initial moniker, the super techno-sounding
“RAD 001”), the metabolic changes seen with these agents,
such as hyperglycemia and hyperlipidemia, may end up challenging
even the most seasoned clinicians.
5. Advances in antiemetics
| 55% |
Percent of oncologists using a regimen with
a second-generation 5-HT3 antagonist to
prevent emesis when prescribing cisplatin/gemcitabine (Figure 7). |
Aprepitant, palonosetron and other 5-HT3 receptor antagonists
have made the use of traditional chemotherapies a less toxic experience
and this has, for example, greatly facilitated the rapidly
emerging use of adjuvant chemotherapy in non-small cell lung
cancer — particularly with cis-based regimens. In our survey,
the dichotomy of how physicians approach premedication with
common regimens such as FOLFOX and GEM/cis suggests that
a significant fraction are either overtreating or undertreating this
classic, highly disturbing, traditional chemotherapy side effect.
6. New chemo regimens: TC (docetaxel/cyclophosphamide) for breast cancer
| 59% |
Percent of oncologists who believe that TC has
a more favorable safety/toxicity profile than
AC (Figure 8). |
We have tracked the TC story since Steve Jones first presented
this important data set at San Antonio several years ago. Steve
was a central figure in the creation of “AC” but now finds it
somewhat amusing and apropos that his new US Oncology study
— at least in his mind — has helped send AC out to pasture.
Dr Jones is pleased that in its place is a less cardiotoxic, leukemogenic and emetic regimen that also seems to be associated
with fewer cancer relapses and resulting deaths, and TC
has been rapidly incorporated into the treatment landscape of
breast cancer.
Questions remain about indications for prophylactic myeloid
growth factors with this regimen, but my sense is that there are
a few too many neutropenic infections out there that might be
prevented. With the recent emergence of the “TIC-TAC-TOE”
trial, it could be that five years from now, TC/bevacizumab
might be the way to go for many patients as adjuvant therapy.
7. New chemo regimens: FOLFOX (oxaliplatin)
| 74% |
Fraction of GI cancer investigators who would
generally recommend FOLFOX to an 84-year-old
patient with Stage III disease and 15/25
positive nodes (27% of practicing docs would
make the same recommendation.) |
| 44% |
Percent of oncologists who start patients on
magnesium and calcium to manage oxaliplatin-related neuropathy (Figure 17). |
I had the good fortune and honor to interview Dr Aimery de
Gramont at the 2003 ASCO meeting, right after he presented
for the first time the MOSAIC trial results, demonstrating an
advantage to FOLFOX compared to 5-FU as adjuvant therapy
for colon cancer. I had held my breath in anticipation of Dr de
Gramont’s arrival at our temporary recording studio in New
Orleans that day, as a fulminant thunderstorm flooded the
streets and pounded the area.
After the soggy but smiling Parisian investigator showed up,
we chatted not only about the MOSAIC efficacy findings with
FOLFOX but also about the reported incidence of neurotoxicity.
A few years later, this critical issue has been muddied by the
confusing sequence of events related to the potential preventive
role of magnesium and calcium. Another issue is the split
between clinical investigators and practicing docs on the use
of this agent in older patients, and new studies attempting to
reduce the number of treatment cycles to six may lead to a considerable
reduction in this important treatment risk.
8. New chemo regimens: Nab paclitaxel
| 27% |
Percent of oncologists who use corticosteroid
premedication with nab paclitaxel (Figure 12). |
| 62% |
Percent of oncologists who believe nab paclitaxel
has a more favorable safety/toxicity profile
than paclitaxel (Figure 13). |
I regularly ask investigators and practicing docs for their thoughts
on this controversial agent. What I have commonly found is that
most physicians — community-based or academic — believe
that in breast cancer, nab offers some advantages compared to its Cremophor®-bound cousin, and if cost and reimbursement
were the same, plain old paclitaxel might have a minimal role in
community practice. What that means and how this information
should be applied to patient care I have no idea, but I am sure that
some health economist somewhere has managed to put a price tag
on the potential avoidance of infusion reactions, insomnia and
agitation. It is also somewhat concerning that a significant fraction
of medical oncologists report using steroid premedications
with this agent, a practice not done in trials evaluating this taxane
and not done by clinical investigators.
9. Targeted adjuvant therapies of breast cancer
(trastuzumab, aromatase inhibitors)
| 65% |
Percent of oncologists who believe that TCH
has a better safety/toxicity profile compared to
an anthracycline-containing anti-HER2 regimen
(Figure 27). |
| 37% |
Percent of oncologists who check cardiac function
six months after initiating adjuvant
trastuzumab (Figure 24). |
The spectacular 2005 ASCO presentations on adjuvant
trastuzumab instantly created 10,000 new cardiologists or,
more specifically, medical oncologists who now had to ramp
up their knowledge base to deal with a serious cardiac threat
for curable patients. The rapid acceptance of TCH (docetaxel,
carboplatin, trastuzumab), which does not seem to increase the
risk of cardiac dysfunction as much as anthracycline regimens,
while providing the same antitumor effect, has resulted in a lot
less stress for patients and oncologists. However, our survey
suggests that docs are being less meticulous about cardiologic
monitoring, maybe because they are less concerned about
complications with no anthracycline involved.
| 73% |
Percent of oncologists who would consider continuing
an AI after five years for a patient who
is tolerating it well (Figure 2). |
| 8% |
Mean estimate by oncologists of the percent
of patients with “severe” arthralgias on AIs
(Figure 1). |
The poor AIs have been getting roughed up a lot in publications
and meetings lately because of their propensity to cause
arthralgias. That may now change instantly with a profoundly
interesting Lancet paper just published by Jack Cuzick and
colleagues demonstrating a fascinating correlation between
vasomotor symptoms and/or arthralgias and relapse rate in
patients treated in the ATAC trial. One wonders if the perspective
on these symptoms might now change in the same manner
as rash with EGFR inhibitors, where docs try to ameliorate this
side effect but encourage patients that this may be a sign that
the agent is working more effectively.
|
