| |
Management of Side Effects Associated with Endocrine
Therapy; Use of Bisphosphonates |
Breast Cancer Update Issue 7, 2007
CHARLES L VOGEL, MD: My experience
with the arthralgias associated with the
aromatase inhibitors has been highly
variable. Approximately 30 percent of my
patients have to be switched to another
therapy or discontinue the aromatase
inhibitor. Aman Buzdar and I had an agreement not to agree. He told me,
“It is a class effect. If you get it with
one aromatase inhibitor, you will get it
with another.” I absolutely do not agree
because I have seen patients respond to a
second aromatase inhibitor.
Breast Cancer Update Think Tank Issue 2, 2008
DANIEL F HAYES, MD: Many of us
underestimated the issue of aromatase
inhibitor-associated arthralgias during
the early clinical trials and when they
were first reported. Increasingly in practice,
many of us are beginning to see
arthralgias as a major issue.
In a prospective trial we have in our
consortium of breast cancer pharmacogenomics,
COBRA, we found in the
first 100 patients we put on a randomized
trial comparing exemestane to letrozole
that 15 percent of patients quit taking
the drug because of joint symptoms.
In a study by Morales and colleagues
reported in the Journal of Clinical
Oncology, they assessed tendon synovial
changes serially with MRI and observed
carpal tunnel thickening in many patients
who were receiving the AIs and in fewer
patients who were receiving tamoxifen.
We don’t know why this happens,
but physicians need to be aware of it. I
have seen a few patients who started out
at baseline with low-level carpal tunnel
syndrome and ended up requiring surgery.
That may have happened anyway,
but it seemed as if it was hastened by the
aromatase inhibitor therapy.
Breast Cancer Update Issue 5, 2008
NANCY E DAVIDSON, MD: Lynn Henry
published data from the first 100 patients
in a clinical trial evaluating the pharmacogenomics
of adjuvant exemestane and
letrozole, and a large proportion saw a
rheumatologist because they crossed a
predefined symptomatology threshold.
The findings are all over the map, and
no single explanation for these symptoms
is clear to me. What to do about them is
also complicated, and one purpose of our study is to determine whether it is possible
to predict which aromatase inhibitor
a patient will tolerate better or perhaps to
identify patients who are more prone to
these musculoskeletal symptoms.
I believe these symptoms were underreported
in the large, randomized
aromatase inhibitor trials. Now that we
are paying attention to this side effect,
we are recognizing that the problem is
critical to address because compliance is
important with these drugs.
Breast Cancer Update Issue 5, 2007
JACK CUZICK, PHD: Not surprisingly, we
see somewhat but not enormously higher
rates of arthralgias with anastrozole than
with tamoxifen in the ATAC trial. The
rate is 30 percent with tamoxifen and 36
percent with anastrozole, so the effect is
real, but it’s a small effect compared to the
fact that arthralgia is not uncommon in
the early postmenopausal years anyway.
So, to some extent, the aromatase
inhibitors are being blamed for some
arthralgias that they don’t cause. They
do increase the risk, but a lot of arthralgias
will occur anyway. We will learn
more about that from the IBIS-2 study
because we’ll be comparing anastrozole
to placebo, and there’s no doubt that a fair amount of arthralgia is occurring in
the placebo arm.
D Cella et al. Quality of life of postmenopausal
women in the ATAC (“Arimidex”,
tamoxifen, alone or in combination) trial
after completion of 5 years’ adjuvant
treatment for early breast cancer. Breast
Cancer Res Treat 2006;100(3):273-84.
These are the first HRQoL data to
become available that cover the entire
5-year treatment period in the primary
adjuvant setting for an aromatase inhibitor.
Although it was not necessarily
expected, results from the 5-year
HRQoL analysis are broadly similar to
those of the 2-year analysis. For both the
anastrozole and tamoxifen treatment
groups, the good HRQoL of patients
at baseline was maintained and perhaps
even improved overall throughout the
treatment period...
Vaginal discharge was less frequently
bothersome with anastrozole but vaginal
dryness, decreased libido, and dyspareunia
were more frequently bothersome
with anastrozole compared with
tamoxifen. HRQoL should play a role in
informed consent and patient-reported
data of this nature add important information
beyond the traditional end points to be considered when making
decisions about therapeutic options and
appropriate supportive measures.
C Derzko et al. Management of sexual
dysfunction in postmenopausal
breast cancer patients taking adjuvant
aromatase inhibitor therapy.
Curr Oncol 2007;14(Suppl 1):20-40.
Clinical evaluation of AI therapy-associated
signs and symptoms of urogenital
atrophy, vaginitis, dyspareunia, and loss of
sexual interest demonstrates several similarities
with natural age- and menopause-related
gynecologic events associated with
diminished estrogen levels. Management
of these events through a combination of
lifestyle modification, counseling, and
hormonal and non-hormonal interventions
can therefore improve quality of life
significantly for patients...
In view of recent findings raising concerns
over elevated circulating estradiol
levels in breast cancer patients on AI therapy
who are using transvaginal estrogenic
preparations, non-hormonal therapies
including regular application of vaginal
moisturizers and lubricants are recommended
and certainly should be first-line
therapy. In addition, pelvic therapy for
pelvic tone awareness and pelvic floor
exercises (for example, Kegel exercises)
and lifestyle modification are preferred
and should be considered early.
Breast Cancer Update Issue 4, 2008
MICHAEL GNANT, MD: The ABCSG-12
trial, evaluating adjuvant endocrine therapies
in premenopausal women, addressed
both the issue of endocrine therapy and
the use of bisphosphonates. The bone
substudy data reported in 2004 revealed
that the bisphosphonates completely
reversed bone loss from aromatase inhibitors. We then increased the trial size from
1,250 to 1,800 to answer the antitumor
question regarding bisphosphonates.
We administered four milligrams of
zoledronic acid every six months, for a
total of seven infusions over three years.
At five years of follow-up, we observed
only 137 disease-free survival events. We
saw a 36 percent improvement in disease-free survival, translating to at least a
nonsignificant trend toward better overall survival. That’s an accomplishment
usually observed with interventions such
as taxane chemotherapy. We observed
that efficacy with an acceptable side-effect
profile.
More importantly, we also saw benefit
in various event subcategories, including
locoregional recurrence, contralateral
breast cancer and distant metastasis outside
of the bone (such as liver or lung
disease). That’s something most of us
did not expect.
When we started the trial in 1999,
nobody was aware of osteonecrosis of the
jaw (ONJ). When the first reports were
published, we made an effort to educate physicians and patients. We identified
three suspected cases and examined the
original dental films. We did not find
evidence of a single case of confirmed
ONJ. This is in line with what is known
about that dose and frequency of administration
of zoledronic acid.
Basically, all the reports suggest that
ONJ with IV bisphosphonates occurs
with more intense regimens or higher-dose
schedules. I would say that ONJ
is not a problem in the adjuvant treatment
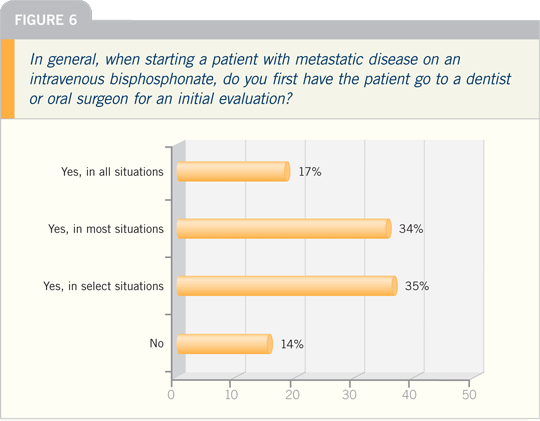
setting. I believe it’s prudent for
patients to see a dentist prior to initiating
bisphosphonate therapy to ensure that
they don’t have any major problems.
DM Reid et al. Guidance for the
management of breast cancer treatment-induced
bone loss: A consensus position
statement from a UK Expert Group. Cancer
Treat Rev 2008;34(Suppl 1):3-18.
In postmenopausal women, the use of
aromatase inhibitors increases bone
turnover and induces bone loss at sites
rich in trabecular bone at an average rate
of 1-3% per year leading to an increase in
fracture incidence compared to that seen
during tamoxifen use...
Randomised clinical trials in postmenopausal
women indicate that
bisphosphonates prevent the bone loss
and accelerated bone turnover associated
with aromatase inhibitor therapy and
are a promising strategy for the prevention
and treatment of osteoporosis in
this setting. Treatment initiation recommendations
are based on a combination
of risk factors for osteoporotic fracture
and BMD levels. Bisphosphonates, along
with a healthy lifestyle and adequate
intake of calcium and vitamin D are the
treatments of choice to prevent bone
loss.
Due to the rate of bone loss associated
with breast cancer treatments,
and uncertainties about the interaction
between aromatase inhibitor use and
BMD for fracture risk, the threshold for
intervention has been set at a higher level
than that generally recommended for
postmenopausal osteoporosis.
Breast Cancer Update Issue 3, 2008
JOHN F FORBES, MD: The data on bone fractures from the long-term follow-up of
the ATAC trial are informative and pleasantly
surprising. For a number of years,
we’ve been aware of the increased risk of
fractures associated with the aromatase
inhibitors compared to tamoxifen. What
was surprising was that upon completion
of the treatment, no difference was
detectable in the risk of fractures with
anastrozole compared to tamoxifen.
It is interesting that no detrimental
carryover effect is evident here. Almost
as soon as you stop the treatment —
within one year — the difference is gone.
I believe we need to be a little cautious
about leaping to safety reassurance at
this point, however, because the types
of fracture risk may vary: Hip fractures
may well be different from vertebral
fractures. These are different types of
bone, and I believe we need much longer
follow-up to be sure that there isn’t some
unsuspected, longer-term effect on hip
fractures.
The bone substudy in ATAC was
designed to evaluate the effect of
anastrozole on bone density and potential
longer-term strategies to correct it.
We learned that women who started out
with a normal bone density may develop
osteopenia but will not develop osteoporosis.
Breast Cancer Update Issue 3, 2007
ANTHONY HOWELL, MD: The important
clinical point from the bone data in
the ATAC trial was that if the patients
started treatment with a normal bone
density, none of them became osteoporotic
over the five years. In addition, we’re
seeing a lot of data on the effectiveness of
bisphosphonates in preventing bone loss
associated with therapy.
The most important data remain those
from the Austrian study, which was published
in the Journal of Clinical Oncology
in 2007. They show that zoledronic acid
at four milligrams administered every six months completely abrogated the bone
loss from goserelin with either tamoxifen
or anastrozole.
Breast Cancer Update Issue 5, 2007
ROWAN T CHLEBOWSKI, MD, PHD: I
believe it’s clear now that almost no one
needs annual bone mineral density testing.
I expect the recommendation will
be every two years. In addition, if the
baseline test is normal and insurance
issues exist, I believe you can wait longer.
As for prophylactic bisphosphonates,
the question is, where do you draw the
line? Some clinicians might choose to
initiate bisphosphonates at a T-score of
-1.5, based on Coleman’s data, and that’s
probably reasonable.
R Weitzman et al. Critical review: Updated
recommendations for the prevention,
diagnosis, and treatment of osteonecrosis of
the jaw in cancer patients — May 2006. Crit
Rev Oncol Hematol 2007;62(2):148-52.
It is recommended that patients
be encouraged to receive a dental examination
prior to initiating bisphosphonate
therapy and, if possible, complete
any necessary dental procedures (eg,
tooth extraction) prior to initiating
bisphosphonate therapy. Patients should
receive regular dental visits during
bisphosphonate therapy.
Patients should be encouraged to
practice good oral hygiene and minimize
possible jaw trauma. If possible, patients
should avoid dental surgery during treatment
with bisphosphonates. If exposed
bone is observed or reported in the oral
cavity at any time (suspected ONJ),
refer the patient to a dental professional
immediately.
Click on the image to enlarge
Select Publications
|
