| |
Dermatologic Toxicities Associated
with Anticancer Treatments |
Lung Cancer Update Issue 3, 2008
THOMAS J LYNCH, MD: We are accruing
to a study evaluating two years of adjuvant
erlotinib for patients with EGFR
mutations (NCT00567359). We know
the response rates in this population
are extraordinarily high. The issue is,
we don’t know what the long-term side
effects are or the optimal duration of
therapy. Patients develop significant
rash. For patients who are really benefiting,
the rash will burn out. It will not
stay at that same level of intensity that
you find in the first two months. In
advanced disease, I have patients who
have been on gefitinib and erlotinib for
four, five, six or seven years.
Breast Cancer Update Issue 2, 2008
NANCY U LIN, MD: Some patients receiving
the combination of capecitabine and
lapatinib report fatigue or mild nausea,
and there is the acneiform rash that is
typical of any of the EGFR inhibitors.
Typically, it appears over the lower part
of the face and the upper chest.
Whether the rash is responding to
treatment or going away on its own is
hard to say, but we’ve used topical antibiotics
with good results.
You definitely see hand-foot syndrome
with capecitabine, and trial data
suggest it may be worse with the addition
of lapatinib.
Breast Cancer Update Issue 3, 2008
DR GRALOW: The rash we have seen
secondary to lapatinib is located on the
face and trunk. My group treats only
patients with breast cancer, so we don’t
have much experience with EGFR-targeted
therapy. We’ve learned of some
topical treatments that we can use. We
don’t usually use oral antibiotics, but we
have done so.
We’re getting better at managing the
rash. From the patients’ standpoint, the
rash is visible. They can tolerate it on
their chest if they can cover it. When it’s
on their face, however, they don’t like to
be labeled or have people ask about it.
Renal Cell Cancer Update Issue 2, 2007
MARIO E LACOUTURE, MD: Dermatological
side effects secondary to sorafenib
and sunitinib are seen with high
frequency. Data from Phase III randomized
studies indicated that sorafenib led
to a hand-foot skin reaction in 30 percent
of patients, with Grade III to Grade IV
severity in only five percent. With sunitinib,
the development of the hand-foot
skin reaction occurred in 20 percent
of patients, and of those cases only five
percent were Grade III to IV in severity.
Hand-foot syndrome also occurs with
other agents, such as fluorouracil or
pegylated doxorubicin. However, these
seem to be clinically and histologically
distinct from the hand-foot skin reaction
occurring with sorafenib and sunitinib.
With more conventional agents, you
have swelling, redness and pain diffusely
through the palms and soles. With
sorafenib and sunitinib, you have a thickening
of the skin. This thickening, when
it is subject to pressure, leads to bleeding
underneath the thickened areas, causing
significant pain for the patient.
Colorectal Cancer Update NSABP Education Session 2006
MICHAEL J O’CONNELL, MD: The toxicity
associated with panitumumab, like
cetuximab, is a cutaneous eruption — acneiform skin rash. Nearly 100 percent
of the patients receiving panitumumab are
reported to have some degree of skin rash.
Infusion reactions have been uncommon
with panitumumab. A variety of other
side effects are seen infrequently — diarrhea,
fatigue — but the major dose-limiting
side effect has been skin rash.

TJ Lynch et al. Epidermal growth factor
receptor inhibitor-associated cutaneous
toxicities: An evolving paradigm in clinical
management. Oncologist 2007;12:610-21.
If patients develop EGFRI-associated
dermatologic toxicity, the following interventions
are suggested, based on severity
of the reaction:
Mild toxicities: Patients may not
require any form of intervention; however,
it may be appropriate to treat some
mild toxicities with topical hydrocortisone
(1% or 2.5% cream) or clindamycin
(1% gel). The EGFRI dosage should not
be altered for mild toxicities.
Moderate toxicities: Treatment is
hydrocortisone (2.5% cream), clindamycin
(1% gel), or pimecrolimus...(1%
cream), with the addition of either doxycycline
(100 mg, po twice a day [bid])
or minocycline (100 mg, po bid). The
EGFRI dosage should not be altered for
moderate toxicities.
Severe toxicities: A reduction in
the EGFRI dose is recommended.
Concomitant intervention is the same as
for moderate toxicities — ie, hydrocortisone
(2.5% cream), clindamycin (1% gel),
or pimecrolimus (1% cream), with the
addition of either doxycycline (100 mg,
po bid) or minocycline (100 mg, po bid)
— but with the addition of methylprednisolone
dose pack. If toxicities do not
sufficiently abate at 2-4 weeks, despite
treatment, then interruption of EGFRI
therapy is recommended, in accordance
with prescribing information.
It is important to note that intervention
for cutaneous toxicities needs to be
maintained even when EGFRI therapy
is decreased or is interrupted, because
EGFRI-associated toxicities may have a
very long duration, analogous to the prolonged
tissue half-life of EGFRIs. Once
the cutaneous reactions have sufficiently diminished in severity, or resolved, then
EGFRI therapy may typically be re-escalated
or restarted with a good degree of
confidence that cutaneous toxicities may
be more easily managed.
Click on the image to enlarge
RTP Satellite Symposium: Molecular
Oncology 101 2008
DR LACOUTURE: I believe that — at
least for most of the EGFR inhibitors
right now — the possibility of having the
discontinuation of these agents and the
toxicities and reverting to a normal state
would be better with the oral agents as
opposed to the monoclonal antibodies.
We do seem to achieve a higher
inhibition of the EGFR pathway with
monoclonal antibodies, which may have
greater antitumor activity by internalizing
the receptor and, therefore, degrading it, and something that can also occur
in skin. That may explain why we see
a 17 to 20 percent rate of Grade III
rash in the patients receiving the EGFR-inhibiting
monoclonal antibodies versus
approximately nine percent with the use
of tyrosine kinase inhibitors.
Might I add that there’s a consistent
story emerging across several classes of
biologics, and that is that a pretty good
link is evident between class-specific toxicity
and efficacy. For example, with
tamoxifen there is now evidence that if a
patient experiences hot flashes, she has a
better likelihood of getting benefit.
We have growing evidence with
VEGF-targeting agents that hypertension
is associated with survival. And, of
course, with EGFR inhibitors, we’ve got
evidence from multiple agents that rash is associated with improved outcome,
so I believe the toxicities are becoming
pretty good pharmacodynamic indicators
of benefit.
Lung Cancer Update Issue 2, 2008
ROMAN PEREZ-SOLER, MD: The
RADIANT study is evaluating adjuvant
chemotherapy followed by erlotinib
administered for two years to
patients with non-small cell lung cancer
(NSCLC) who have EGFR-positive
disease as determined by IHC or FISH.
The issue will be whether a patient can
receive erlotinib for two years — if that
would be tolerable. I believe it will be
tolerable for most patients.
The first two months may be rough,
but after two months of erlotinib, the
majority will find that the toxicity subsides and the skin rash improves. A
minority will need a dose reduction or
will not be able to tolerate the drug.
Click on the image to enlarge
RTP Satellite Symposium:
Molecular Oncology 101 2008
DR VENOOK: There’s an adjuvant study
(NCCTG-N0147) evaluating FOLFOX
with or without cetuximab for patients
with Stage III colon cancer. That is a
tough sell. These patients believe they
are cured. They’re hedging their bets by
taking more therapy, and we have trouble
accruing patients and many drop out
because they don’t want a rash. I believe
their perception of the gain and what’s
at stake is, as with most decisions by
patients, the important factor.
However, in the advanced disease setting,
patients tend to be much more forgiving
of these kinds of toxicities. The
perversion, of course, is the correlation
with the degree of skin toxicity and efficacy
of the agent. That may be favorable
from a prognosis perspective, so in the
waiting room, patients actually compare rashes and they are pleased when they
have a bad rash.
DR LACOUTURE: With regard to the
dermatologic problems that patients who
are receiving cetuximab may encounter, I
believe that by instituting early intervention
and frequent follow-up, we can maintain
the majority of patients on therapy,
and that is associated with their overall
sense of well-being and quality of life.
The two randomized trials in which
the administration of tetracycline antibiotics
was administered prophylactically
showed a significant benefit. In
one study, tetracycline at 500 milligrams
twice daily showed a significant reduction
in Grade II or worse rash.
A second trial evaluating oral minocycline
at 100 milligrams twice daily
for cetuximab-associated rash, published
in the Journal of Clinical Oncology in December 2007, showed treatment
reduced the number of lesions.
Renal Cell Cancer Update Issue 2, 2008
ROBERT A FIGLIN, MD: We participated
in the study that evaluated sorafenib in
older patients with advanced renal cell
carcinoma and found no apparent difficulty
administering sorafenib to patients
older than age 65 compared to younger
patients.
In my experience, older patients tolerate
sorafenib better than sunitinib. We
see less fatigue, hand-foot syndrome and
hypertension with sorafenib.
I believe that some of the problem with
the hand-foot syndrome that’s seen, specifically
with the tyrosine kinase inhibitors,
is that we don’t realize how much we
traumatize our hands and feet every day
through our normal activities and that
angiogenesis is part of wound healing.
When we inhibit angiogenesis and
inhibit wound healing, we also inhibit
the ability of these hands and feet to get
better. That’s why, when you stop these
drugs for a period, the hands and feet get
better quickly.
The single most important management
strategy that my nurse tells me
about all the time is anticipating the
toxicities before they occur. We need to
let patients know what they may experience when to call and then what to do.
They should not wait until the toxicity
is so robust that the only alternative is to
stop the drug.
Once the patient is experiencing them,
the easiest way to manage toxicities such
as hand-foot syndrome is to stop the drug,
restart at a lower dose and recognize that
we may be able to escalate the dose later.
However, the further the toxicity has
developed, the longer the patient will be
off of treatment before it reverses.
Renal Cell Cancer Update Issue 2, 2007
DR LACOUTURE: For patients who are
receiving sorafenib and sunitinib, the
hand-foot skin reaction tends to develop
after the first month of therapy. With
sorafenib, for which an administration
of 400 milligrams twice daily is uninterrupted,
you tend to see it earlier than
with sunitinib, as the sunitinib regimen
allows for a two-week drug holiday.
Patients are able to recover from the
tenderness and pain during that two-week
drug holiday.
Flushing — the red face and the
seborrheic dermatitis-like reaction —
occurs within the first two to four weeks.
Hand-foot skin reactions usually occur
later, and they tend to become worse over
time if the symptoms are not managed.
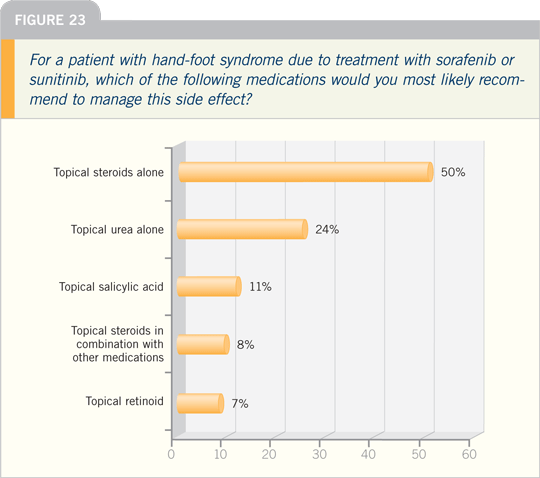
For management, we have used high-concentration
urea-containing preparations,
such as urea 40 percent creams.
These are keratolytics, so they disrupt
the outer layer of the skin, the stratum
corneum. They seem to thin out that
thickened skin layer that may be responsible
for the increased pressure leading
to the pain.
We also prescribe high-potency topical
steroids, such as clobetasol ointment,
as this will minimize the proliferation or
the division of those skin cells. It will also
decrease the underlying inflammation.
Select Publications
|
