| |
Cardiotoxicity, Thrombosis and Bleeding Associated
with Anticancer Treatment Regimens |
Special Edition Breast Cancer Update:
Cardiologic Issues in Breast Cancer
Management 2008
JEAN-BERNARD DURAND, MD: Symptoms
of heart failure and the side effects
of cancer treatment can be similar. As a
result, physicians may be missing signs
of cardiotoxicity with only history and
physical examination.
In 2004, an interesting study was published
in the Journal of Clinical Oncology that examined patients who had a constellation
of eight symptoms and their physicians’
ability to recognize these adverse
events. It showed that 75 percent of the
time, physicians did not pick up on what
the patient was feeling. We might, therefore,
have to rely on studies such as biomarkers,
echocardiograms and MUGAs
to better detect these symptoms.
The recommendations from a number
of societies for patients receiving cardiotoxic
drugs include an echocardiogram at
baseline and when a change in symptoms
occurs, such as lower extremity edema.
On physical exam, physicians should
watch out for elevated neck veins, lower
extremity edema, an S3 on exam or bilateral
rales. The problem is that the sensitivity
of these physical exam findings is
low. The accuracy of diagnosing heart
failure based on something as simple
as bilateral lower extremity swelling is
approximately 35 percent.
The best clinical predictor we have
found has to do with weight gain. We
teach patients about the “rule of twos,”
which is if while on therapy they put on
more than two pounds within two days,
to contact us. We’re watching for an
early sign of fluid retention, and we treat
that aggressively. Ultimately, that keeps
patients on track so that they do not have
to discontinue their cancer therapy.
Special Edition Breast Cancer Update:
Cardiologic Issues in Breast Cancer
Management 2007
DENNIS J SLAMON, MD, PHD: In
the adjuvant trastuzumab trials, we
frequently saw patients with HER2-positive disease who were treated with
AC but never received the taxane/trastuzumab therapy because they had
declines in their LVEFs, yet those are not
scored as toxicities.
It’s a greater negative when that happens
than when other things happen
because now you’re denying a potentially
active drug to a woman who might benefit
from it because you forced the agenda
with the anthracycline. That happened
between three and five percent of the
time in all the studies that were examined,
and it’s more frequent among older
patients. The problem is that once physicians
see the LVEF drop, they’re reluctant
to take the risk.
I believe that oncologists are becoming
increasingly aware that the cardiac
toxicities might continue for longer than previously believed. The assumption had
been that once we stopped trastuzumab,
the cardiac problems reversed in a matter
of a couple of weeks or months. However,
the data — at least the BCIRG 006 data
— show that they are longer lasting. We
now know that a year and a half later,
those subclinical LVEF declines seem to
be maintained at some level.
We previously thought that the
patients with clinical congestive heart failure
improved with treatment. However,
at least two thirds of them require continued
treatment. That means that you
can treat their congestive heart failure,
but it doesn’t mean that you’ve made the
heart better. I believe that these definitions
must be more carefully stated when
some of the data are presented.
Special Edition Breast Cancer Update:
Cardiologic Issues in Breast Cancer
Management 2008
HAROLD J BURSTEIN, MD, PHD: Monitoring
ejection fractions in patients on
adjuvant trastuzumab and deciding
when to stop and start the agent is a
difficult situation because we have a
black-box warning about cardiotoxicity
with the use of trastuzumab.
Clearly, these patients merit cardiac
surveillance. It seems as if borderline
ejection fraction at baseline, age and
perhaps preexisting hypertension stand
out as predictors of trastuzumab-related
cardiomyopathy.
The patients still require surveillance,
irrespective of those risk factors. My practicing
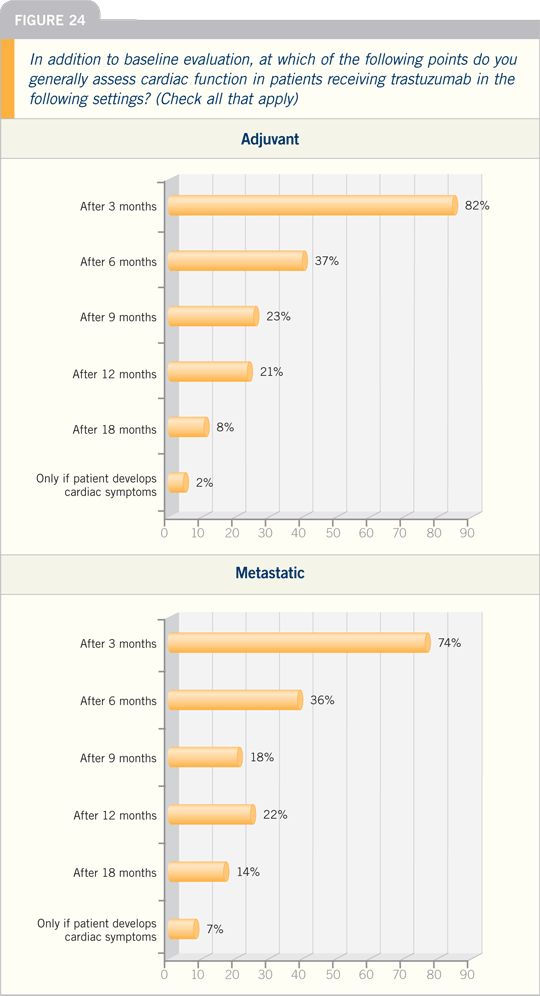
algorithm is to check cardiac function
at baseline, after the anthracycline-based
chemotherapy, after three to four
months of the taxane/trastuzumab combination
and at some point again. It must
be said that these safeguards were put in
place when we did not know the clinical
efficacy of trastuzumab.
The challenge arises in cases with
high-risk breast tumors in which you
are trying to bring important therapy to
bear on the patient’s disease. When you
are trying to combat these reductions
in ejection fraction of unknown clinical
significance, it’s tough to be a clinician
because we have no hard and fast rules.
The rules in the trials were based on not
knowing that trastuzumab would be a
lifesaving drug for women.
Special Edition Breast Cancer Update:
Cardiologic Issues in Breast Cancer
Management 2008
DR DURAND: The use of trastuzumab
has shifted to earlier and earlier and
despite that, the safety data remain good.
The data from the clinical trials show
the incidence of heart failure is low —
two to four percent — and we saw few
deaths. The morbidity was higher than
anticipated, but at the time these trials
were conducted, we weren’t administering
ACE inhibitors and beta blockers or
trying to track these events as secondary endpoints. I believe that with medical
intervention, the incidence of heart failure
would probably be even lower.
In addition, we know that the reversibility
of the trastuzumab-induced cardiac
damage is excellent. At our institution,
we have seen clinically that when we
put these patients on beta-blockers and
ACE inhibitors, they have an excellent
ability to completely recover their normal
heart function.
We presented a paper at the Heart
Failure Society of America in 2002 on
young women who were asymptomatic
but developed small drops in heart function.
Their heart function was in the
range of 40 to 50 percent.
The patients wanted to remain on the
trastuzumab because they knew it reduced
their rate of disease progression by 50 percent,
so we spoke with their oncologists
and we put them on both an ACE inhibitor
and a beta-blocker without stopping
the trastuzumab. We never stopped the
trastuzumab, their heart function went
back to completely normal and our longest
follow-up is now five years.
Breast Cancer Update Issue 6, 2008
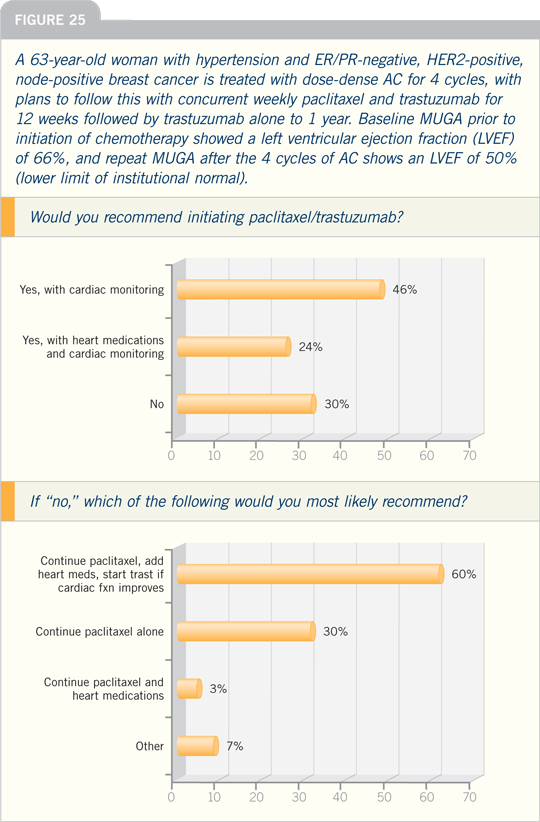
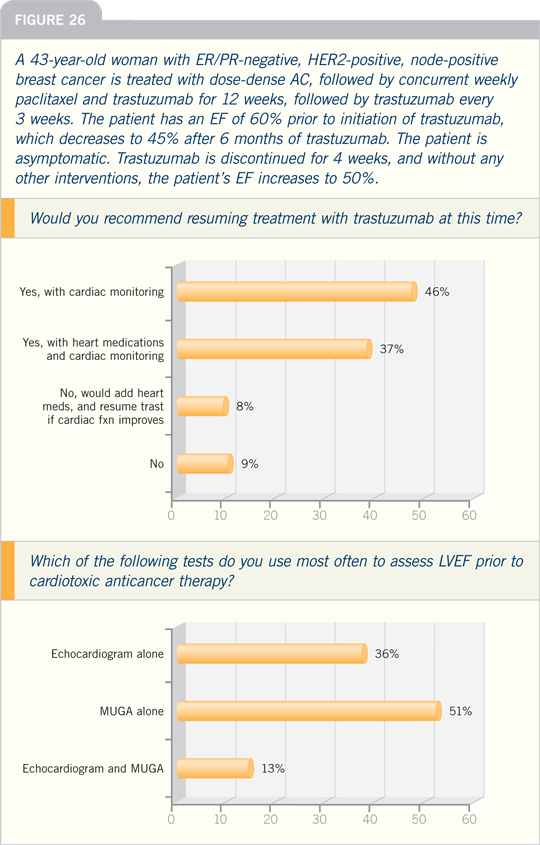
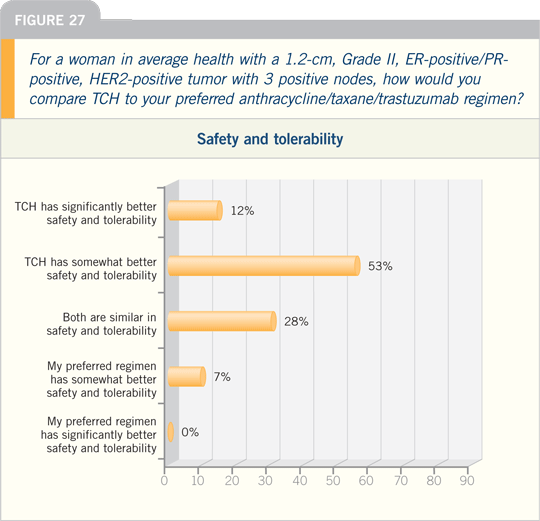
DR PICCART-GEBHART: The issue of
an anthracycline-versus a nonanthracycline-containing chemotherapy for a
patient with HER2-positive, node-positive
disease is a hot topic. In Europe, we
are selecting the type of chemotherapy
based on risk factors for cardiotoxicity,
including age, obesity, poorly controlled
hypertension and a left ventricular ejection
fraction that is on the low end of the
normal range prior to initiating therapy.
For patients who are at a higher risk
for cardiotoxicity, it is reasonable to
choose a nonanthracycline-based chemotherapy.
I prefer TCH, the regimen
that has been piloted in the BCIRG 006
study. It is important to be able to clearly
explain to patients the side effects they
can expect with this regimen.
For a 38-year-old woman who has five
positive nodes but is in otherwise perfect
health, we have two options. The five positive
nodes are worrisome and indicate a
higher risk for an early relapse. You do not
want to give a six-month chemotherapy regimen and then start trastuzumab. It
makes sense for such a woman to go with
TCH or what we like to do in Europe,
which is three cycles of FEC — this is
anthracycline-based but only three cycles
— and then move on to a taxane, which
can be docetaxel or paclitaxel, administered
concomitantly with trastuzumab.
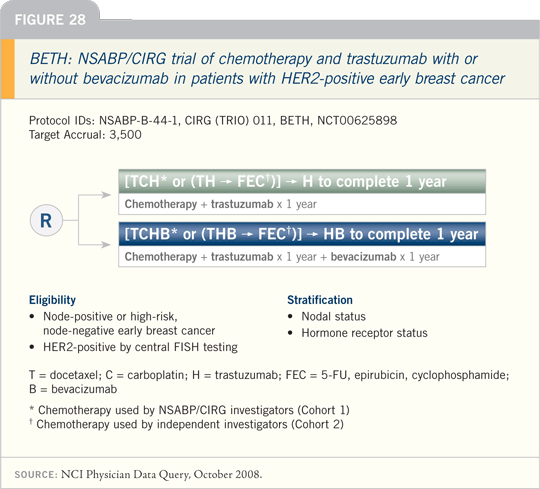
Breast Cancer Update Issue 5, 2008
DR WOLMARK: The BETH study
— the adjuvant trial being conducted
by the NSABP and CIRG evaluating
trastuzumab with or without bevacizumab
— opened recently (Figure 26). I
believe we need to know what the addition
of bevacizumab to trastuzumab will
yield in the adjuvant setting, based on
some interesting preclinical work and
early clinical findings. Cardiovascular
concerns exist with both agents, so the
NSABP and the CIRG are offering
TCH as the template.
We made the decision not to use an
anthracycline template to test the combination
of trastuzumab and bevacizumab,
with one of the rationales being the
potential toxicity of using both agents on
an anthracycline template.
However, some participating physicians,
particularly those in Europe,
will administer an anthracycline template
along with bevacizumab and
trastuzumab, so I believe we will receive
an answer rapidly as to whether that
regimen is tolerable.
Special Edition Breast Cancer Update:
Cardiologic Issues in Breast Cancer
Management 2008
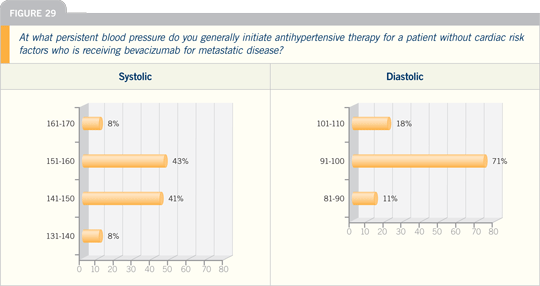
DR DURAND: At our institution, with
bevacizumab we have a recommendation
that the patient’s blood pressure must be
less than 140/90 for treatment, and that
is incorporated into all our clinical trials.
We have follow-up data that show our
incidence of heart failure is actually quite
low, less than two percent, if we control
the patient’s blood pressure more aggressively.
We are finding that we are able
to keep women involved in these newer
trials that include trastuzumab and bevacizumab
on therapy much longer if we
just do a better job of internal medicine.
Click on the image to enlarge
Renal Cell Cancer Update Think Tank
Issue 1, 2008
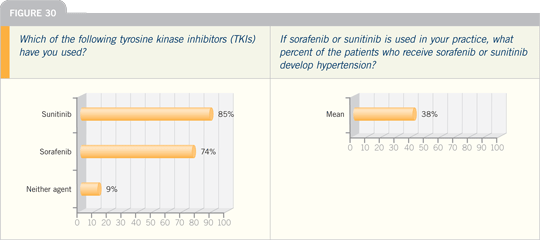
WALTER STADLER, MD: I want to
emphasize that we cannot minimize
some of the cardiovascular toxicities of
the multikinase inhibitors, sunitinib
and sorafenib, in renal cell carcinoma.
The rate of hypertension in the clinical
trials is approximately 20 percent,
and increases in blood pressure occur
in probably two thirds to three fourths
of patients. We’re talking about chronic
treatment with these agents — perhaps
years of treatment when we use these
agents sequentially — and we’re talking
about an elderly population, which may
have multiple comorbidities, including
cardiovascular disease.
We observe cardiovascular events,
even with short follow-up, in the current
Phase III trials — an increased risk
of cardiovascular and cerebrovascular
events. Attending to the cardiac toxicities
from these agents will be an increasingly
important issue in terms of patient care.
I tell patients that a risk of a cardiovascular
or cerebrovascular event exists
on the order of about three percent, in
comparison to one percent in controls in
the randomized trials.
Lung Cancer Update Issue 3, 2008
DR LYNCH: It appears that anticoagulation
therapy can be part of the approach
to lung cancer in patients who are receiving
bevacizumab. It’s more difficult to
come by data on patients who are receiving
anticoagulants prior to therapy. I have
been hesitant to use bevacizumab in that
setting. I’m not simply concerned about
the anticoagulants — I’m concerned about
why they were receiving these agents.
Most of my patients who are anticoagulated
have experienced an acute
thrombotic event. I’ve been hesitant to
use bevacizumab for the patient with a
pulmonary embolism discovered at diagnosis,
who has an acute need for heparin,
whereas for the patient who has been
receiving warfarin for a long time for
atrial fibrillation, I suspect it’s fine to use
bevacizumab. The concern is more for
the patient who’s been acutely anticoagulated
for a clotting event such as a deep
vein thrombosis, pulmonary embolus or
a myocardial infarction.
Colorectal Cancer Update for Nurses
Issue 1, 2007
DR HALLER: The issue of wound healing
in patients receiving bevacizumab
is subjective. Surgeons evaluate wound
healing every day, and I suspect if they
knew their patient was on bevacizumab,
they might say the healing was slower
than usual. No huge difference exists
in operative complications in patients
receiving bevacizumab. The agent has
a long half-life of more than 21 days,
so even when the patients are six weeks
out, they still have enough bevacizumab
in their system to inhibit anything. The
truth is that sometimes people need to
go to surgery and don’t delay surgery just
because they received a dose of bevacizumab
two weeks ago.
A paper was published on patients who
underwent liver resection after receiving
six cycles of chemotherapy and bevacizumab,
but the bevacizumab was held
during the last cycle so that four weeks
had elapsed between surgery and the last
dose. The data revealed no obvious difference
in regeneration of liver or bleeding, so while I believe that it’s an issue theoretically,
and probably somewhat real,
in practice it doesn’t seem to be a “deal
breaker.” Nor is it a major issue for minor
but necessary surgical procedures, such as
insertion of a port or dental surgery.
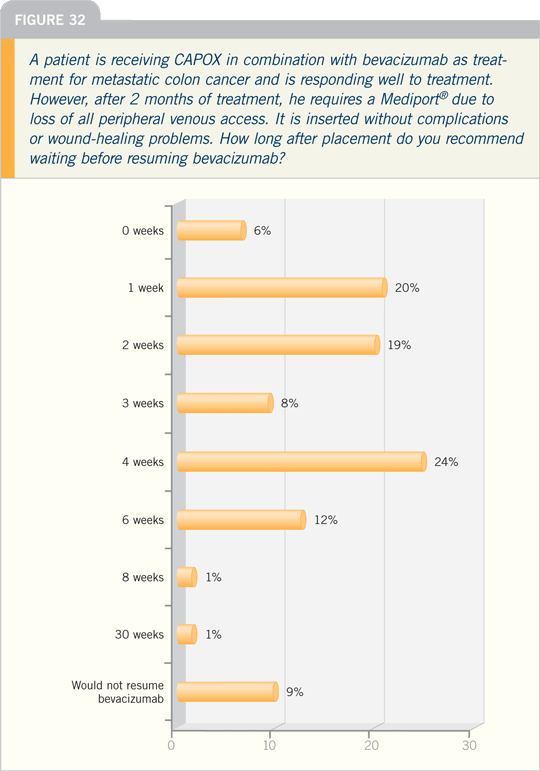
Lung Cancer Update Issue 3, 2008
F ANTHONY GRECO, MD: Most of our
protocols require a week between putting
a port in and using bevacizumab. Personally,
I have done it the same day for
several patients, and so far I’ve not seen a
problem. That’s not a huge incision and
it doesn’t go through viscera.
I certainly wouldn’t use bevacizumab
within a week after bowel resection.
Sometimes patients who have received
bevacizumab then have an emergency
in which they need surgery and we don’t
have a choice. Interestingly enough, most
of those patients fare well, but I don’t
tempt fate and undertake major surgery
by design after proximate use of bevacizumab.
Lung Cancer Update Issue 2, 2008
JULIE R BRAHMER, MD: With regard
to predictors of hemoptysis secondary to
bevacizumab, there are many thoughts.
Some believe the location of the tumor is
important — central versus peripheral.
Others believe a history of hemoptysis
increases a patient’s risk. Still others feel
it is related to the presence of tumor cavitation,
which is borne out in a small analysis
by Dr Sandler. Therefore, if tumor
cavitation is present initially, I generally
avoid using bevacizumab.
If the tumor develops cavitation while
responding to bevacizumab, some recommend
stopping bevacizumab. Some recommend
using radiation therapy in that
local area to try to decrease the risk of
bleeding with bevacizumab. If cavitation
did occur with therapy, particularly in a
central lesion, I would seriously talk with
the patient about the increased risk.
I don’t believe location is quite as
important, because bevacizumab has been
administered to patients with small cell
lung cancer. Those tumors are all mainly
central, and we’ve seen no increased risk of
bleeding in that patient population.
Lung Cancer Update Issue 3, 2008
DR LYNCH: The eligibility criteria for
the AVAiL trial and ECOG-E4599 did
not restrict tumor location, and in subsequent
reviews, central tumors didn’t
appear to be a problem. So in my practice,
having a tumor that’s central or
abutting the pulmonary artery or aorta,
in and of itself, doesn’t mean that patient
can’t receive bevacizumab.
However, I believe we are learning
that pretherapy cavitation may be significant.
One of the big challenges now
is how to manage tumor cavitation that
develops in response to therapy. In a way,
that’s what you’re hoping for because
those cavitary responses are some of the
best responses we see. However, they
are associated with an increased rate of
hemoptysis, which is of concern.
In my practice, when a patient’s tumor
has an enormous cavitary response, I generally
stop the chemotherapy and bevacizumab
and observe that patient. That
is completely unevidence based — I’m simply nervous in that setting. I believe
we need to spend more time examining
exactly how those patients fare.
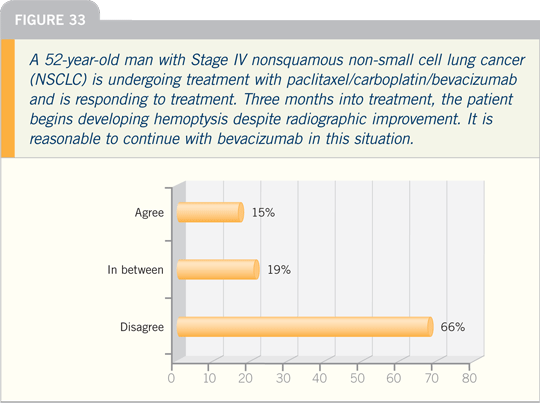
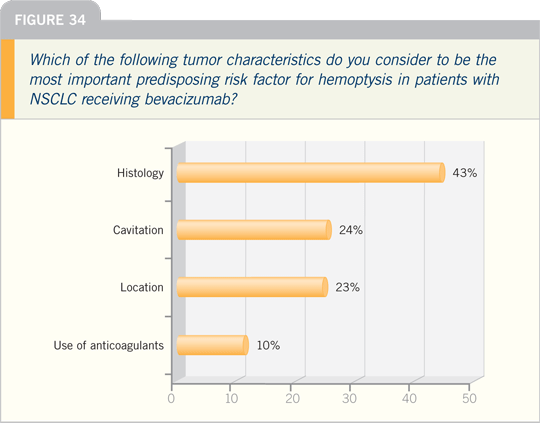
Select Publications
|
