| |
Treatment of Chemotherapy-Associated Side Effects |
A Naeim et al. Evidence-based recommendations
for cancer nausea and vomiting. J Clin
Oncol 2008;26(23):3903-10.
There are many types of neuroreceptors
that are involved in the emetic response,
including serotonin (5-hydroxytryptamine-3 [5-HT3]), dopamine, corticosteroid,
and neurokinin-1 receptors;
therefore, antiemetic agents often target
different neuroreceptors and can behave
synergistically when used in combination.
Without the use of prophylactic antiemetic
therapy, some highly emetic types
of chemotherapy, such as cisplatin, would
almost universally result in nausea and/or
vomiting, but with the use of optimal
antiemetic therapy, clinicians can reduce
the prevalence to approximately 25% of
patients on highly emetic therapy. Therefore,
appropriate pharmacologic prevention
and management are essential.
PJ Hesketh. Chemotherapy-induced
nausea and vomiting. N Engl J Med
2008;358(23):2482-94.
Four groups (the Multinational Association
of Supportive Care in Cancer, the
American Society of Clinical Oncology,
the National Comprehensive Cancer
Network, and the European Society
for Medical Oncology) have recently
published updated antiemetic guidelines.
There is broad agreement among
these groups on most key issues. The
treatment recommendations that follow
reflect a composite of the consensus
recommendations of these groups...
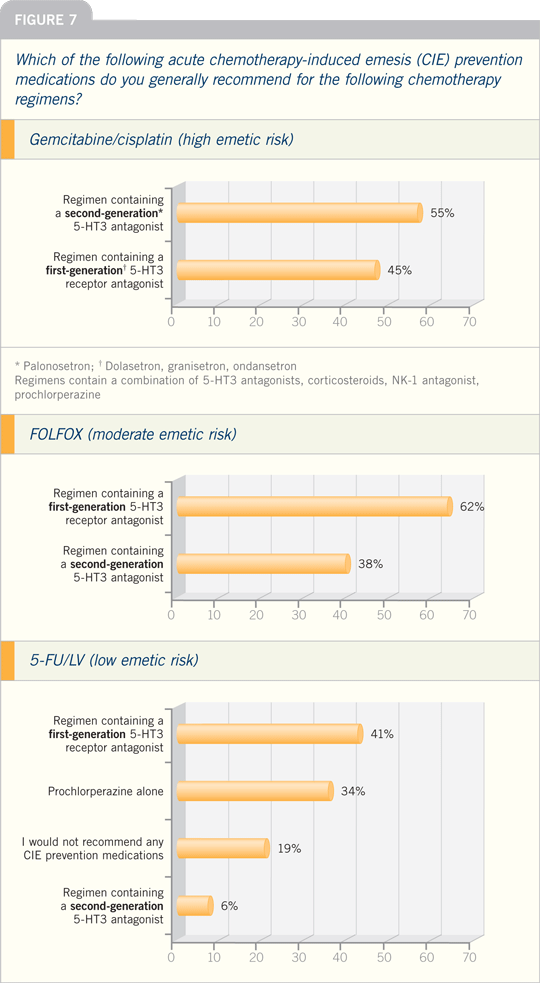
High Emetic Risk
The combination of a 5-HT3 antagonist,
dexamethasone, and aprepitant is
recommended before the administration
of chemotherapy that is associated with
a high risk of emesis... Patients receiving
chemotherapy with high emetogenic
potential should receive a combination
of aprepitant on days 2 and 3 and
dexamethasone on days 2 to 4...
Moderate Emetic Risk
In patients receiving treatment with an
anthracycline and cyclophosphamide,
a combination of a 5-HT3 antagonist, dexamethasone, and aprepitant is recommended
before chemotherapy. Because
this chemotherapeutic regimen has a
moderate potential for delayed emesis,
aprepitant should also be administered
on days 2 and 3...
Low Emetic Risk
A single dose of dexamethasone before
chemotherapy is recommended for
agents associated with a low risk of
emesis. A single dose of a dopaminergic
antagonist is another reasonable preventive
option. No routine prophylaxis for
delayed emesis is indicated...
Minimal Emetic Risk
No routine prophylaxis for acute or
delayed emesis is warranted for chemotherapeutic
agents that are associated
with a minimal risk of emesis.
Breast Cancer Update Issue 2, 2007
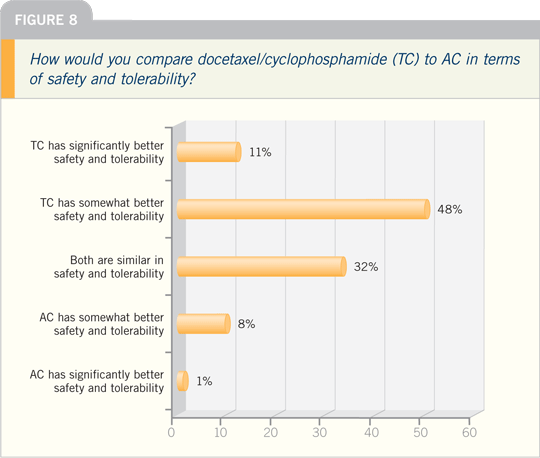
FRANKIE A HOLMES, MD: I’ve started
to incorporate the TC regimen much
more frequently in my practice, especially
in situations in which I have concerns
about chemotherapy tolerance. However,
at this time, I have not given up on
the standard AC taxane regimen for my
patients with node-positive disease. AC
is now recognized as a highly emetogenic
regimen, and patients may experience
delayed nausea and vomiting. I was once
on a panel discussing emesis, and someone
said, “Oh, that’s just AC.” AC is associated
with a lot of delayed nausea and
vomiting. You find considerable hidden
toxicity if you step into the shoes of a
patient. It can be incapacitating. With
TC, you don’t have that level of burden of
emesis and nausea.
Breast Cancer Update Issue 2, 2008
STEPHEN E JONES, MD: We examined
the database from the US Oncology
adjuvant trial evaluating TC versus
AC for long-term potential toxicities
and identified three fatal events: congestive
heart failure in a woman younger
than age 50, myelodysplastic syndrome
and myelofibrosis. Those three patients
received AC chemotherapy, and we saw
nothing similar in the TC arm.
These are the concerns with
anthracyclines. They adversely affect the
heart, a fact that has been underappreciated.
We are beginning to understand
this effect better, particularly in older
patients. Data from MD Anderson and
the SEER and Medicare databases demonstrate
that the occurrence of congestive
heart failure may be in excess of 10 or 20
percent among women older than age 65
when treated with anthracyclines.
That’s scary, and I wonder, did I contribute
to this? It would be nice to have
a treatment that eliminated doxorubicin, which may be responsible for some of the
late congestive heart failures.
The anthracyclines also increase nausea
and vomiting. In our original report,
significantly less Grade III/IV nausea
and vomiting was recorded with TC
versus AC, and more antiemetics had to
be used for those patients with delayed
nausea and vomiting.
NCCN Clinical Practice Guidelines in
Oncology: Antiemesis — v.3.2008
The development of the 5-HT3-receptor
antagonists (such as ondansetron, granisetron, dolasetron mesylate, palonosetron)
represents a significant advance in antiemetic
therapy. All of these agents have
been shown to be effective in controlling
the acute nausea and/or vomiting associated
with cancer chemotherapy.
Palonosetron is a 5-HT3 antagonist
with an approximately 100-fold higher
binding affinity for the 5-HT3 receptor
compared to the other serotonin antagonists
(ie, ondansetron, granisetron, and
dolasetron). It has a half-life of approximately
40 hours, which is significantly
longer than other commercially available
5-HT3 antagonists... It is recommended
(category 1) for acute and delayed emesis
prevention when using moderate emetic
risk chemotherapy...
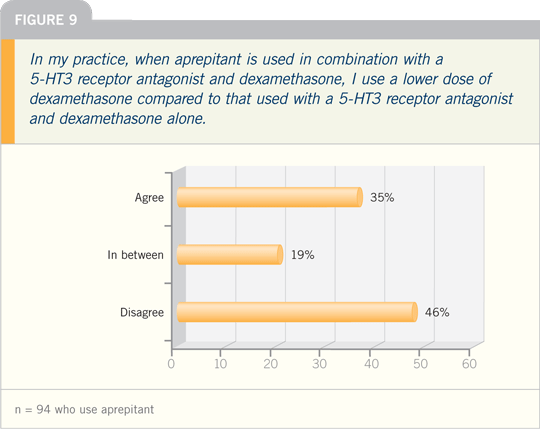
In March 2003, the Food and Drug
Administration (FDA) approved aprepitant
(oral), which selectively blocks the
binding of substance P at the NK-1 receptor
in the central nervous system. Thus,
aprepitant provides a different and complementary
mechanism of action to all
other commercially available antiemetics.
Aprepitant has been shown to augment
the antiemetic activity of the 5-HT3
receptor antagonists and the corticosteroid
dexamethasone to inhibit both acute
and delayed cisplatin-induced emesis.
Breast Cancer Update Issue 3, 2008
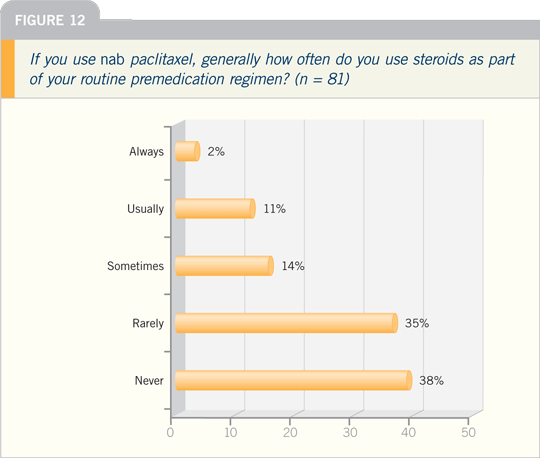
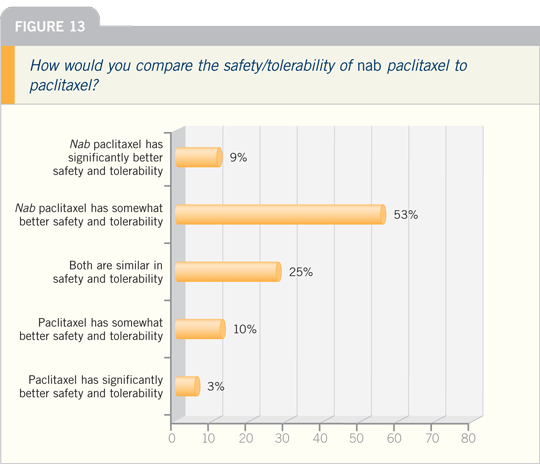
JULIE R GRALOW, MD: Nab paclitaxel
does not require premedications, has a
faster infusion time and has the ability
to deliver somewhat higher doses
of the drug. I believe we are seeing a
dose-response effect above what we’ve
traditionally observed with paclitaxel.
Certainly the data with every three-week
nab paclitaxel versus paclitaxel are in
favor of nab paclitaxel.
We have randomized Phase II data
showing that when administered weekly,
nab paclitaxel may be as good as, if not
better than, docetaxel. It’s a fascinating
drug, and I like using it a lot. I like not
having to administer steroids and antihistamines
and the markedly reduced
chance of allergic reactions. I’m excited
about trials moving nab paclitaxel into
the adjuvant setting.
Breast Cancer Update Issue 2, 2007
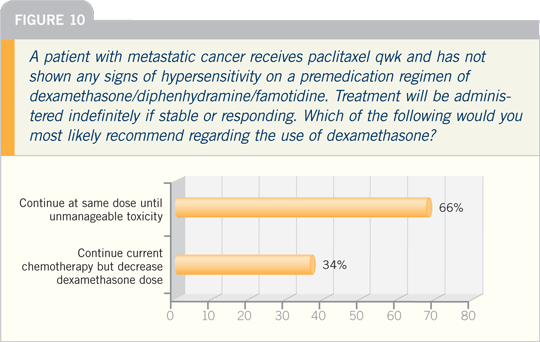
DR HOLMES: I administer nab paclitaxel
in preference to paclitaxel, period, for
patients with metastatic disease. It’s
a huge advantage. Patients have a life.
They have kids. They have day care.
At its best, getting through the clinic
is difficult. I have emergencies, so I’m backed up. There are all kinds of built-in
delays.
We all think we know what it is to
go through therapy from the patient’s
standpoint, but it’s hard to remember
all the delays, all the problems: “Oh
gosh, counts aren’t up today. You have
to come back later.” To begin with, these
people have a life, and their time is valuable.
In addition, not having to take the
dexamethasone is a huge benefit. How
many people are hyperactive? They can’t
sleep that first night. They have to take
the steroids and then take lorazepam or
something else to relax them. Finally, we
all know that patients gain weight on
adjuvant chemotherapy.
Apparently, they do not eat more, and
their energy intake isn’t increased, but
their energy expenditure is decreased.
Add this anabolic agent on top of that,
and we know that some women are sensitized
to this. Then there’s that minority
of patients who develop acne from the
dexamethasone. Really, less is more, and
avoiding premedication is a tremendous
advantage.
Breast Cancer Update Issue 2, 2008
JOYCE O’SHAUGHNESSY, MD: One of the
main advantages of nab paclitaxel is that
you don’t need steroids. Steroids weren’t
used in the nab paclitaxel trials, and an
increasing body of anecdotal evidence
suggests that patients who suffer reactions
with paclitaxel or docetaxel can
receive nab paclitaxel without having
anaphylactoid problems. I don’t know
of any reason to administer steroids to
them.
Breast Cancer Update Issue 5, 2007
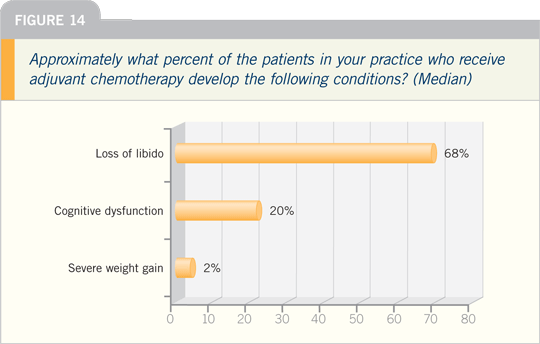
SHARON GIORDANO, MD, MPH: We’ve
conducted exploratory work examining
cognitive dysfunction secondary to
chemotherapy. It’s difficult to get information
from databases because most of
the treatment-related cognitive changes
are subtle, and I don’t believe most physicians
would notice them during a routine
office visit.
The cognitive changes are important
to the patient in terms of memory-only span or recall, attention span, but these
are picked up on careful neurocognitive
testing.
Clearly, a number of issues exist in
the patient’s life at the time of diagnosis
and treatment, including an enormous
amount of stress, possible cytokines
released by the cancer that may cause
cognitive changes even before starting
chemotherapy, the effects of chemotherapy,
the effects of menopause in women
and the effects of estrogen blockade. So
many different things are going on that
it’s hard to tease out how much each contributes
to the dysfunction.
It clearly is a real phenomenon for
some patients. One of my young patients
in her thirties received chemotherapy,
then had difficulty remembering people’s
names at the day care center she operates.
When I saw her a year later at follow-up,
it seemed to be getting better. I believe
real changes occur, but only some patients
are strongly affected in this way.
Colorectal Cancer Update Issue 3, 2008
STEVEN R ALBERTS, MD: The
CONcePT trial was designed to evaluate
intermittent versus continuous oxaliplatin,
with chemotherapy, and the use of
calcium and magnesium to decrease oxaliplatin-induced peripheral neuropathy.
The Data and Safety Monitoring
Committee halted the trial based on an
early analysis that suggested calcium and
magnesium were causing some detrimental
effect in terms of the response rate, as
well as potentially progression-free survival.
An independent review group examined
the outcomes and it now appears that
patients receiving calcium and magnesium
were not harmed by it and, indeed,
they seem to have a better response rate
and a longer duration of disease control.
However, because the trial was
stopped early, there’s still some concern
regarding whether we can rely on the
data. Meanwhile, the North Central
Cancer Treatment Group (NCCTG)
was evaluating calcium and magnesium
in a symptom-control trial. The analysis
from that trial also suggested there
wasn’t any potential harm in terms of
response rates. In addition, the NCCTG
and the CONcePT trials both showed
some benefit from calcium and magnesium
in controlling treatment-induced
peripheral neuropathy.
Colorectal Cancer Update Issue 5, 2007
HERBERT I HURWITZ, MD: One approach
to maximize the treatment benefit from
oxaliplatin in the metastatic setting is
to be preemptive through the use of a
calendar schedule. This is the OPTIMOX
approach, by which stopping and
starting treatment are based as much on
the calendar as they are on the patient’s
symptoms or disease control.
I find that adjustment based on the
patient’s symptoms — as long as the
threshold of symptoms is lowered —
ends up being a nearly identical approach.
I have a bias to try to adjust based on how the patient is faring rather than
the calendar, but in practice, the two
approaches are most likely similar.
Currently my algorithm is to reduce
or stop only the problem agent and to
continue the portions of therapy that
seem to help, as long as they’re well tolerated.
For patients who need a break for
personal reasons or for asthenia, I believe
stopping even the fluoropyrimidine and
bevacizumab for a period of several weeks
to two months is a reasonable approach,
as long as the disease burden and patient
symptoms allow for the holiday.
Click on the image to enlarge
Select Publications
|
