| |
Treatment of Small Cell Lung Cancer |

Lung Cancer Update 2006 (3)
DR HANNA: The Japanese Cooperative
Oncology Group (JCOG) reported a
positive Phase III study in small cell
lung cancer (SCLC) four years ago evaluating
the combination of irinotecan
and cisplatin compared to a control
arm of etoposide and cisplatin, and the
etoposide and cisplatin arm performed
as you would expect. The irinotecan
arm was statistically superior. The study
was meant to accrue approximately 225
patients, but the Data Safety Monitoring
Committee stopped the study early,
according to the statistical design, based
on the positive findings. So only 150
patients were accrued.
We set out to either confirm or refute
those data in a largely US patient population.
We used cisplatin and etoposide
as our control arm. We modified the
dose and schedule of the irinotecan arm.
Thirty percent of the patients on the
JCOG trial never received their day-15
irinotecan. We were hoping to make an
every four-week regimen an every three-week
regimen, and therefore, you would
intensify the dose.
We also sought to take advantage of
the synergism between irinotecan and
cisplatin, so we split the dose. When you
administer cisplatin at its full dose and
irinotecan at its full dose, you see quite a
bit of nausea and vomiting. So the hope
was, by splitting the dose, it would be
more tolerable.
I was lucky enough to present the data
at ASCO last year. It involved approximately
330 patients. It was a two-to-one
randomization. Approximately 220
patients received irinotecan and cisplatin,
which represents three times the number
of patients who received irinotecan
on the JCOG trial. Unfortunately, we
weren’t able to replicate the data. The
efficacy parameters were all the same.
The median survival was approximately
nine and a half months to 10 months on
both arms. The one-year survival was the
same on both. The differences between
the regimens were largely in terms of the toxicities. The etoposide arm caused
more neutropenia and more neutropenic
infection. The irinotecan arm caused
more diarrhea and mucositis and dehydration.
So it is a trade-off of side effects.
You have to think about the individual
patient. You have to determine which
side-effect profile you should consider
for your individual patient.
The Southwest Oncology Group is
replicating the JCOG regimen. Both
arms of the SWOG-S0124 study are
identical to the arms of the JCOG study.
It’s a much larger trial than the JCOG
trial, larger than our trial, with more
than 500 patients. I understand that its
accrual is quite good. If it’s a matter of
our changing the dose and schedule of
the irinotecan arm, and that was why
it was not superior, then the Southwest
Oncology Group study should show us
that.
Lung Cancer Update 2006 (4)
DR THOMAS E STINCHCOMBE: We’re
interested in investigating nab paclitaxel
in patients with small cell lung cancer.
In our Phase I trial, we saw some nice
responses in patients who had been previously
treated for SCLC. The advantage
of the combination of carboplatin and
nab paclitaxel for patients with SCLC
would be a reduction in febrile neutropenia.
Our current regimen of cisplatin/irinotecan is associated with a significant
incidence of febrile neutropenia of
approximately five percent. If we could
administer carboplatin/nab paclitaxel
every three weeks, it would be a significant
improvement in terms of patient
convenience over cisplatin/etoposide
or carboplatin/etoposide on days one
through three.
Cancer Conference Update 2007 (3)
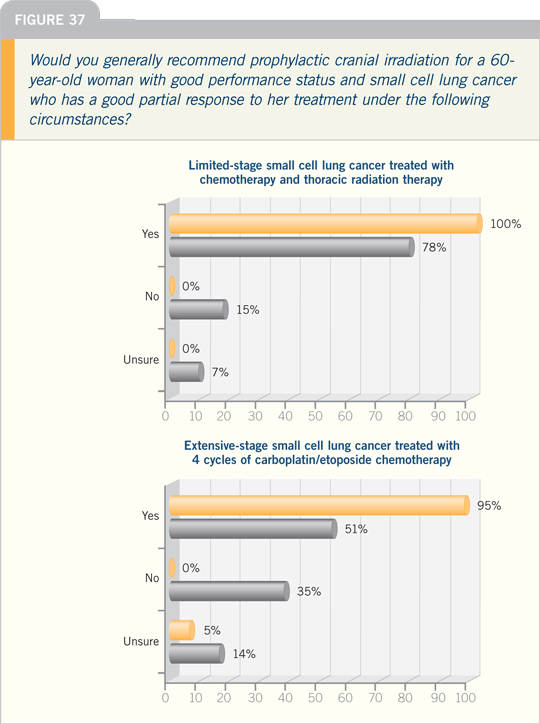
DR KIM: Brain metastases are a big
problem in SCLC. A meta-analysis
published in 1998 suggested a decreased
risk of brain metastases and an
improvement in survival with radiation
therapy. This was predominantly in
limited-stage SCLC with some extensive-stage small cell disease.
The EORTC study that was presented
at ASCO 2007 on the use of
prophylactic cranial irradiation (PCI) in
extensive-stage SCLC was interesting.
I believe we have to take the data with
a grain of salt in that they show some
proof of concept but, again, we have to
tailor the data to our patients in practice.
This study focused on extensive-disease
SCLC. Four to six cycles of therapy were
administered up front. They called this
induction therapy but, in fact, it is the
routine therapy we administer for extensive
disease. The patients, if they experienced
any response — and this response
was gauged by the investigators or the
treating physicians, it was not based
on RECIST — were then randomly
assigned to receive PCI. The PCI varied
between 20 and 30 Gray in a one-week
or two-week time frame, or no PCI.
Randomization occurred within five
weeks of completing the chemotherapy,
and then patients were required to start
the PCI within six weeks of completing the
chemotherapy.
The primary endpoint was to demonstrate
a reduction in risk of developing
symptomatic brain metastases, and the
key word here is “symptomatic.” That
goes with the spirit of the entire study — patients were responding. They may
have been symptomatically responding,
feeling better. It’s a palliative situation.
There was not a mandated staging
of the brain at baseline, which can obviously
be quite problematic. We had a
list of eligibility criteria, by which the
patient had to have one or more of the
listed symptoms in order to require an
imaging test, either a CT or an MRI. We
don’t know what would have happened if
they had all undergone imaging — some
of those people might have had gross disease
already but been asymptomatic.
The numbers were quite positive. The
hazard ratio for development of symptomatic
metastases was 0.27. It’s nearly
a 75 percent reduction, which was a very
favorable outcome. One-year survival
was approximately double with PCI and
was reported as 27 percent versus 13.3
percent. Failure-free survival was 23.4
versus 15.5 percent. And again, some
stratification issues that were different
regarding the amount of extrathoracic
disease and extracranial disease existed
in the two cohorts. So I don’t believe,
definitively, that we can say one way or
another that every patient who responds
to chemotherapy should receive PCI.
I don’t believe that this is that kind of
study. But for providing a proof of principle,
it validates the concept that administering
PCI to patients with extensive-stage
SCLC and good chemotherapy
responses may be justifiable.
Lung Cancer Update 2007 (1)
DR BELANI: When you compare SCLC
to non-small cell, you don’t see the same
patient numbers and morbidity and
mortality. But if you compare it to other
tumors, then it’s a significant disease.
There is not enough research on it.
Among all lung cancers, the numbers
have dropped from 20 percent to 13
percent. Most of them are being treated
in the community because the response
rate with standard treatments is high
enough that these patients don’t show
up for a research study. Therefore, it
will be difficult to compare experimental
regimens to the standard regimen
in the front-line setting. We need to
develop select markers for select patients
in the second-line and recurrent disease
settings and take them to the front-line
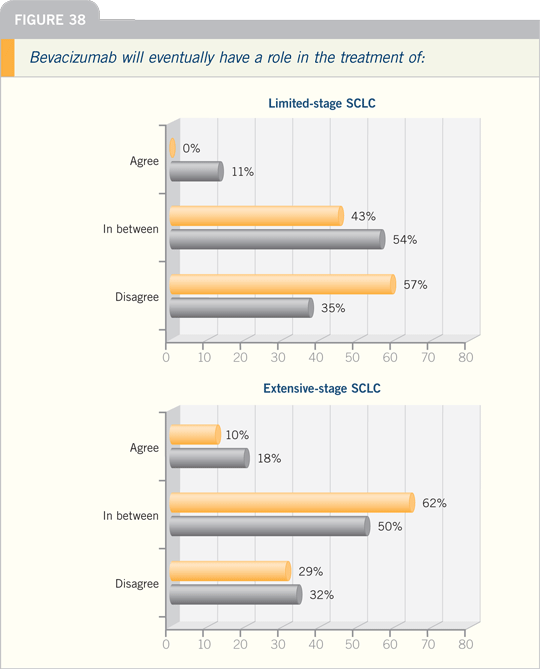
setting. I believe bevacizumab is one of a
class of VEGF-targeted compounds that
still should be evaluated in SCLC.
Lung Cancer Update 2007 (2)
DR SCHILLER: ECOG completed a
Phase II trial in extensive-stage SCLC,
which was platinum/etoposide and bevacizumab.
It was a one-arm trial that
had only 68 patients. It met its first
safety endpoint, and the study itself was
completed approximately six months ago.
There were no unusual toxicities and,
specifically, there was a lack of hemoptysis.
We are planning to move forward
with the randomized Phase III study
of cisplatin/etoposide with or without
bevacizumab in extensive-stage disease
through ECOG.
Select Publications
|
