| |
Treatment of Stage III Non-Small Cell Lung Cancer |
Lung Cancer Update 2006 (4)
DR PASS: The question of how
to treat Stage IIIA lung cancer
has been a vexing one. A number of
studies have been performed using
induction therapy for Stage IIIA
nodal disease, two of which, despite
very small accrual, were highly touted
for the positive survival advantage seen
among patients who received induction
cisplatin-based therapy.
By the same token, Phase II trials
studying the combination of chemotherapy
and radiation therapy resulted in a
randomized trial that evaluated whether
induction chemoradiation therapy was better
than definitive chemoradiation therapy
without surgery for Stage IIIA disease.
The RTOG-9309 study presented
by Dr Kathy Albain at ASCO 2003
appeared to suggest that surgery after
induction chemoradiation therapy
was not any better than definitive
chemoradiation therapy, although it was
associated with a trend toward improved
progression-free survival.
If you evaluate the data carefully,
however, you notice a high mortality rate
for patients who underwent pneumonectomy.
The overall operative mortality
rate was seven percent, but the operative
mortality rate in patients requiring
pneumonectomy was 14 percent.
A subsequent unplanned analysis of
the trial was presented by Dr Albain at a
follow-up ASCO 2005 meeting, in which
the authors carefully matched patients
treated with definitive chemoradiation
therapy to patients with lobectomies
and not pneumonectomies.
Sure enough, they found a fairly dramatic
survival advantage in the lobectomy-only group favoring combined
chemoradiation therapy with surgery.
Click on the image to enlarge
Lung Cancer Update 2007 (4)
DR HANNA: In 2003, SWOG published
results from the SWOG-S9504 Phase
II trial. The study included 83 patients
with Stage IIIB disease who were treated
with cisplatin/etoposide for two cycles
concurrently with 61 Gray of radiation,
followed by three cycles of consolidation
docetaxel.
The median survival time was 26
months. This patient population should
have had a median survival time of about
13 months with Stage IIIB and chemoradiation
treatment only. Instead, they
had a five-year survival of 29 percent.
Historically, that group should have had
a five-year survival of five, seven, eight
percent.
This engendered a lot of enthusiasm
and became a de facto standard for many
physicians, based upon a single, relatively
small Phase II trial. We sought to confirm
that this strategy was effective. We
did a randomized Phase III study that
included patients with both Stage IIIA
and Stage IIIB disease.
A total of 243 patients entered our
trial. All patients received cisplatin/etoposide and concurrent radiation with
59.4 Gray. Then, after a rest period of
four to eight weeks — and as long as they
had not progressed and remained eligible
— patients were randomly assigned to
either three cycles of docetaxel consolidation
or observation.
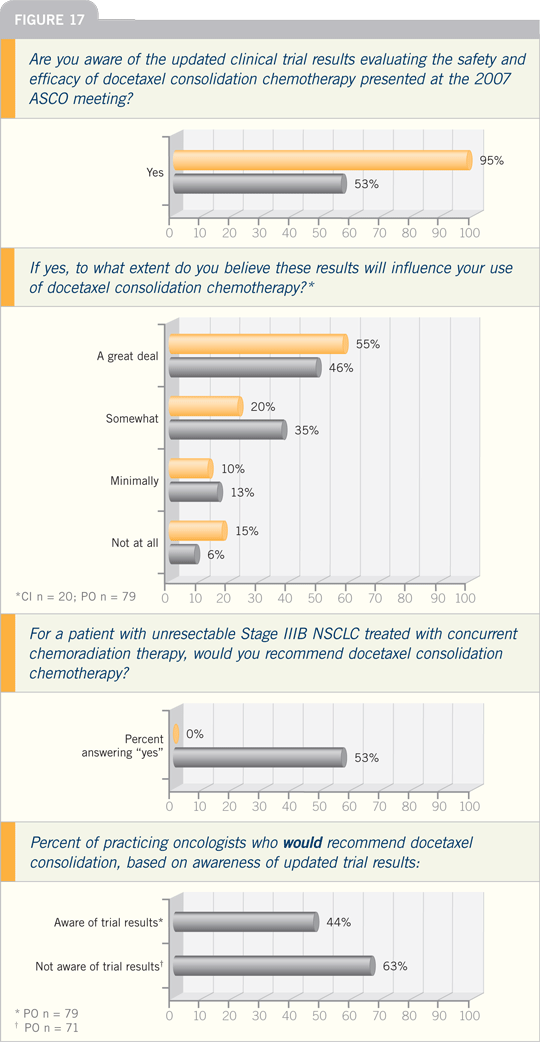
We reported several provocative
findings. No difference in progression-free survival between the two
randomized arms was seen, and there
was no difference in median survival,
three-year survival or overall survival.
The p-value was 0.9. The curves were
completely superimposable.
Lung Cancer Update 2007 (3)
DR WAKELEE: The Hoosier Oncology
Group (HOG) trial, which evaluated chemotherapy with cisplatin/etoposide
and concurrent radiation therapy with
or without consolidation chemotherapy
for unresectable Stage IIIA and IIIB
disease, was the most practice-changing
presentation in lung cancer at ASCO.
All patients in the study received chemotherapy
and radiation therapy, and then
they were randomly assigned to either
consolidation docetaxel using the standard
SWOG-S9504 protocol or nothing.
The trial showed no difference in
survival between the two arms.
Criticisms include the fact that it was
a relatively small study and it was stopped
early because of an interim analysis
showing that there was no way statistically
to obtain a separation of the curves.
The study begs the question of what
consolidation chemotherapy is achieving
in that situation.
Other studies that evaluated induction
chemotherapy with additional
chemoradiation therapy in a similar
patient population also didn’t show any
benefit beyond the standard chemoradiation
intervention. Again, it’s bringing
into the forefront this question of what
to do with Stage III disease.
For several years, everyone has been
comfortable with the SWOG-S9504
regimen. Now we have to question that.
However, I have a hard time believing
that two cycles of a platinum doublet
with radiation therapy are enough to cure
Stage III disease when we know we need
more than that to improve survival for
earlier stages. I don’t believe the question
is dead, but I believe we need to move away from simply building on S9504.
Many people are still using a weekly
carboplatin-based regimen and a taxane
with the radiation therapy. To say that we
shouldn’t administer any chemotherapy
after that is a somewhat frightening proposition,
considering that these patients
are not receiving much chemotherapy at
all during the radiation therapy.
Select Publications
|
