| |
Adjuvant Therapy of Non-Small Cell Lung Cancer |

Click on the image to enlarge
Lung Cancer Update 2007 (1)
DR CHANDRA P BELANI: Adjuvant
therapy has become the standard for
patients with resected non-small cell
lung cancer. After 2005, it was the standard
for patients with Stage IB to IIIA
disease. Recently we have developed a
brewing controversy regarding whether
we should administer adjuvant therapy
to patients with Stage IB disease.
One issue in the controversy is
whether or not it was carboplatin that
caused the failure of the carboplatin
and paclitaxel regimen for patients with
Stage IB disease in CALGB-9633. At
long-term follow-up, the data failed to
show an improvement in overall survival
because the hazard ratio fell from 0.62
to 0.80 and the p-value was no longer
significant. As a word of caution: This
was a small trial, and it is still not completed.
In general, considering the results
of the other clinical trials, the JBR.10
study, the IALT study and the ANITA
trial, adjuvant chemotherapy did play
a role in Stage IB disease, but in those
trials the chemotherapy was cisplatin
based.
The CALGB-9633 trial has shown
in a subset analysis that among patients
who have tumors greater than four centimeters,
a benefit still exists. But again,
we may be reading too much into these
subset analyses, which were not clear endpoints
of these clinical studies.
In the clinical setting, for Stage IB
disease, I offer chemotherapy to patients,
informing them that in a small subset it
has shown a benefit and in another subset
it has not shown a benefit. I let the
patient decide whether he or she wants
to receive adjuvant chemotherapy. If the
tumor is greater than four centimeters
in size, then I usually suggest that the
patient receive it.
Lung Cancer Update 2007 (4)
DR MARTIN J EDELMAN: I believe the
weight of data supports a cisplatin-based
adjuvant regimen. If one wants
to be completely data driven, cisplatin/vinorelbine is probably the most validated
regimen out there, but it’s difficult to
administer. In Stage IV disease, cisplatin/docetaxel is at least as good — possibly
even superior — and probably better
tolerated than cisplatin/vinorelbine, so I
consider that a reasonable regimen.
If somebody told me that he or
she intended to administer cisplatin/vinorelbine, I would not argue about it.
Click on the image to enlarge
Click on the image to enlarge
The combination of cisplatin/gemcitabine
is also a reasonable approach. I
believe the crucial drug in this regimen
is the platinum agent.
However, despite all the arguments,
I believe carboplatin/paclitaxel is also
reasonable. It has been pointed out that
to conduct an adequately powered study
of patients with Stage IB disease, you’d
have to enroll about 2,000 patients.
So the CALGB carboplatin/paclitaxel
study that showed an improvement in progression-free survival in Stage IB
disease was probably underpowered.
If you consider the subgroup of patients
with tumors measuring four centimeters,
you see that those patients clearly
fared better. I don’t believe carboplatin/paclitaxel is inactive in this setting —
occasionally we do use that combination.
Why? We use it because some patients
simply cannot tolerate cisplatin-based therapy.
It is not unusual for us to start with
a cisplatin-based therapy and then switch
the patient after one or two cycles because
he or she cannot tolerate it. So for their
final couple of cycles, these patients are
treated with a carboplatin-based therapy.
Click on the image to enlarge
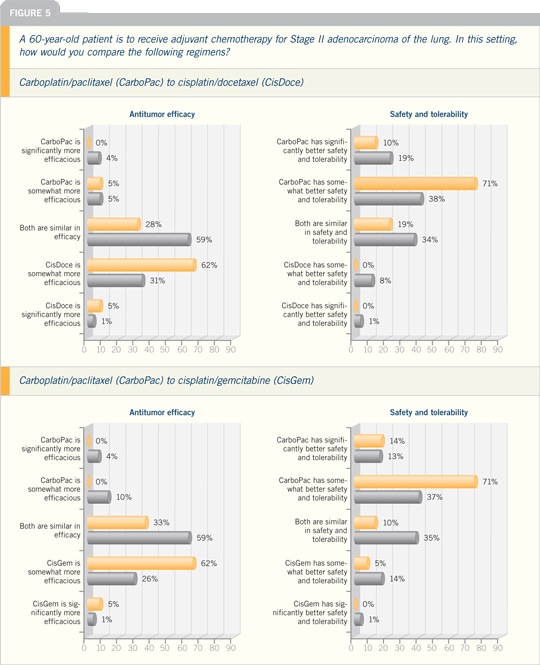
Interview, July 2007
DR NASSER H HANNA: I use cisplatin-based
adjuvant therapy, unless the patient
has a contraindication for cisplatin. If a
patient has modest renal insufficiency,
I’ll administer carboplatin. I believe
the general practice in the oncology
community is to use carboplatin-based
therapy. However, I think little difference
in outcome is likely to appear.
We had a clue about that from ASCO
2007, when Milleron presented an early
analysis of a neoadjuvant trial. He indicated
that a regimen of carboplatin/paclitaxel
resulted in the same degree of success
as cisplatin/gemcitabine — in terms of
complete resection rate, response rate
and percent necrosis — suggesting that
a carboplatin-based neoadjuvant regimen
may be as good as cisplatin. We don’t have
any survival data on that study yet.
I generally combine docetaxel with
cisplatin. The majority of data we have
from the adjuvant setting is with cisplatin/vinorelbine. But multiple trials
have been conducted comparing cisplatin/docetaxel to cisplatin/vinorelbine
or single-agent docetaxel to single-agent
vinorelbine, in which docetaxel is a more
active and effective agent.
Lung Cancer Update 2007 (3)
DR MARK A SOCINSKI: We are currently
conducting a Phase II study evaluating
docetaxel and carboplatin in the adjuvant
setting.
We previously conducted a feasibility
study of that combination, and our
endpoint was to determine whether
we could deliver four cycles of therapy
within 12 weeks to more than 80 percent
of the patients.
The study included 72 patients and
showed that 80 percent of them were
able to receive four cycles. We allowed
patients to receive growth factor support,
and approximately one third of the
patients received growth factors at some
point during the four cycles.
No treatment-related deaths occurred.
Our conclusion was that this is a feasible
regimen for the patient whom you consider
not to be a good candidate for a
cisplatin-based approach. The Phase II
safety data suggest that you can use that
regimen. The data in our trial were similar
to what the CALGB showed with
carboplatin and paclitaxel.
Click on the image to enlarge
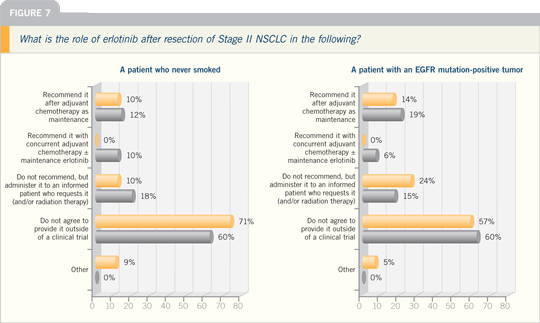
Lung Cancer Update Think Tank 2007
DR THOMAS J LYNCH: My use of
neoadjuvant therapy has declined over
the years.
I had a lot more enthusiasm for it
before we had evidence that adjuvant
therapy had benefit. The only patients
for whom I tend to think of neoadjuvant
therapy now are those with bulky N2
disease, for whom we will administer
neoadjuvant chemotherapy up front and
proceed to surgery.
Adjuvant therapy has completely
changed the dimension of neoadjuvant
therapy. I don’t see a great advantage to
neoadjuvant therapy over adjuvant therapy
for Stage I and Stage II disease. Even
for patients with resectable Stage IIIA
disease, neoadjuvant therapy is questionable
at that point.
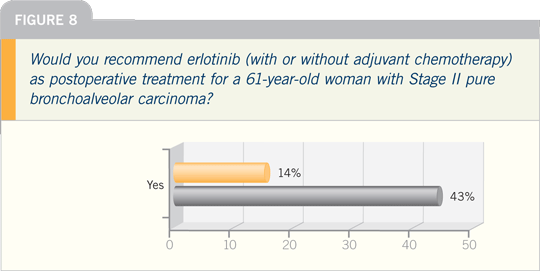
Lung Cancer Update Think Tank 2007
DR HANNA: I can think of a couple of
patients for whom we have used preoperative
therapy recently.
Some patients have lost weight, and
you become nervous about moving them
to surgery up front, but you believe the
disease is still curable radiographically
— bulky N2 disease — and ultimately,
you know they will require both surgery
and chemotherapy. So you administer a
few courses of chemotherapy and allow
the disease to declare itself.
Lung Cancer Update 2006 (4)
DR VINCENT A MILLER: We now have
several markers that can predict benefit
from EGFR TKIs in the metastatic
setting, which can be determined in
any patient — such as smoking history,
ethnicity and pathology — and some in the molecular arena.
In the arena of clinical variables, factors
include never smoking, adenocarcinomas
and Asian ethnicity. I believe
a history of never smoking is the most
powerful predictor of benefit.
ASCO 2006 was important in
terms of reporting some prospective
trials of EGFR tyrosine kinase
inhibitors in patients known to have
EGFR mutations. The lowest response
rate in prospectively identified patients
with mutations was about 65 percent,
and it went up to about 85 or 90 percent.
So a patient has about a 75 or 80 percent
chance of having a response if he or she
has an EGFR mutation. That is pretty
good compared to what we had two or
three years ago and even compared to
what we have in other commonly studied
diseases that are driven by diagnostic
testing.
In our trial for patients with bronchoalveolar
cancer — presented at ASCO
2006 — we had some patients with an
EGFR mutation and a high EGFR copy
number. Their response rate was 90 percent
and their median survival was about
three years with erlotinib.
The response rate for patients without
an EGFR mutation and with an EGFR
copy number lower than four was four
percent, and their median survival was
only 15 months. Those are pretty powerful
predictors for a difference in clinical
outcome.
Lung Cancer Update Think Tank 2006
DR HARVEY I PASS: For the patient who
is a never smoker or has an EGFR mutation,
I have to say that I can’t, off trial,
dissuade him or her from adjuvant erlotinib
because it makes sense to me.
Obviously, the trial must be performed
to answer the clinical question: If
we compare erlotinib with the best adjuvant
chemotherapy regimens, is that the
way to go? I believe we’re talking about a
selected population. In that situation, I
can’t go against the patient who has read all the data and wants to go that route.
DR LYNCH: I’ve softened on this issue.
I believe for patients who have mutation-positive disease, you need to have a
detailed discussion with them. They’re
not going to be able to wait for the Phase
III trials to be conducted, and obviously,
I endorse the concept of Phase III trials.
However, for that patient with mutation-positive
disease, I have a long discussion
with them, and I don’t believe it’s crazy
to consider adding erlotinib after chemotherapy.
Lung Cancer Update 2007 (3)
DR BRUCE E JOHNSON: The adjuvant
setting is more complicated, and it will
be a long time before we know how to
use assays for EGFR and incorporate
them into the therapeutic algorithm. The
trial currently being planned will evaluate
patients with the epidermal growth factor
present either by immunohistochemistry
receptor or by fluorescence in situ hybridization.
After surgical resection, those patients
who are believed to benefit from chemotherapy
will receive adjuvant therapy.
If the tumor is EGFR-positive by
immunohistochemistry or fluorescence
in situ hybridization, those patients will
be randomly assigned to receive erlotinib
versus placebo (Figure 9).
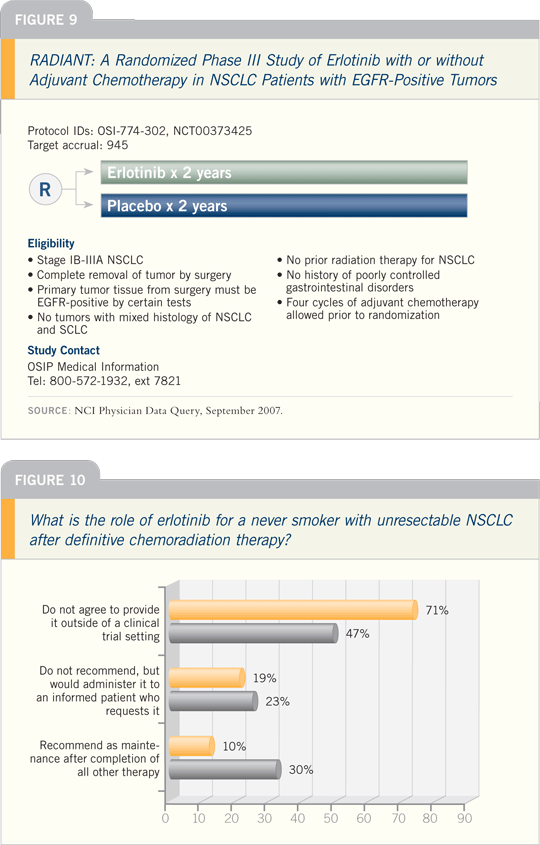
That trial will take several years to
accrue the patients, and because it’s in
patients with relatively early-stage disease,
we will have to wait for three to five
years from the time the last person is
enrolled to see the survival information.
So we don’t know that just yet.
The other part that’s embedded
within that trial is that those patients
will also be studied for other determinants
of benefit, including the mutations
of the epidermal growth factor receptor.
The amount of tumor tissue available
for these adjuvant studies is obviously
much greater than in studies of patients
with advanced disease, with whom we’re
typically working with needle aspirations
or bronchoscopic biopsies.
Lung Cancer Update 2007 (3)
DR SOCINSKI: In the absence of data and
in the wake of SWOG-S0023, in which
the use of an EGFR TKI after chemoradiation
therapy showed a decreased
survival rate, I have been conservative
in my approach. I administer adjuvant
chemotherapy — I have not yet administered
adjuvant erlotinib or gefitinib.
There is an outstanding prematurely
stopped trial by the NCI of Canada, in
which adjuvant gefitinib was studied in
unselected patients. It was closed before
it met its accrual goal, and we don’t have
any information yet.
We currently have an adjuvant
trial, RADIANT (Figure 9), which
selects patients with EGFR-positive disease
by immunohistochemistry or FISH analysis, in which you can administer
chemotherapy or not. Then they’re randomly
assigned to a placebo or erlotinib.
Until we see data from that trial, I have
not used it as a recommended treatment
in the adjuvant setting.
Lung Cancer Update 2007 (2)
DR COREY J LANGER: In the ECOG-E4599
first-line advanced NSCLC
study, the addition of bevacizumab to
paclitaxel/carboplatin demonstrated
a two-month improvement in median
overall survival and about a six to eight
percent improvement in one- and two-year
survival. It also showed more toxicity,
particularly pulmonary hemorrhage.
In the bevacizumab/paclitaxel/carboplatin arm, 15 treatment-related
deaths occurred out of 305 patients. Not
all were related to hemorrhage — some
were from neutropenic fever or other
causes.
In the control group, two treatment-related deaths occurred out of
344 patients. So, although we excluded
patients with squamous histology, brain
metastases, ongoing thromboembolic
phenomena, anticoagulation use or antecedent
hemoptysis, we still saw a heightened
treatment-related death rate.
I believe many of those concerns are
going to fall by the wayside in the adjuvant
trial. The tumors have been resected.
By definition, these patients have no
residual tumor in the chest. Ideally, they
should not have pulmonary hemorrhage.
Lung Cancer Update 2006 (3)
DR EDWARD S KIM: Bevacizumab works
well in the metastatic setting, so there is a
rationale to move our best metastatic regimen
to adjuvant therapy.
With bevacizumab, you need to consider
the problems that could occur in a
postoperative setting. We have to derive
that from the colon trials. We’re not sure
if there will be any wound dehiscence
in lung cancer patients who have had
surgery.
Lung Cancer Update 2007 (3)
DR HEATHER A WAKELEE: We activated
the ECOG-E1505 (Figure 12) study
recently and are more comfortable
than ever with our choice of regimens
that investigators can select: cisplatin/gemcitabine, cisplatin/vinorelbine and
cisplatin/docetaxel, all with and without
bevacizumab.
Click on the image to enlarge
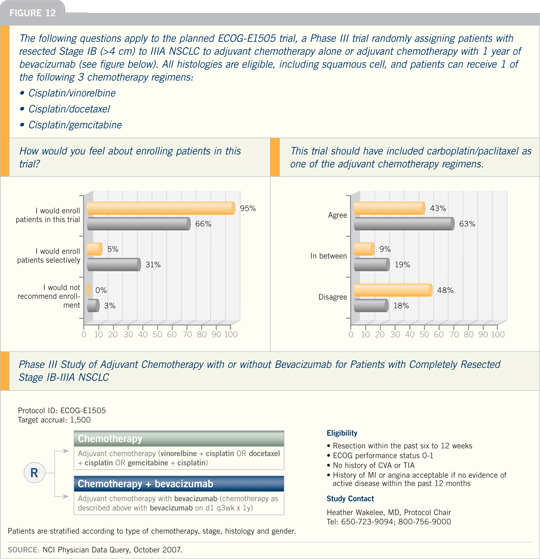
At this point, we’re still sticking with
the 15-mg/kg dose of bevacizumab
because that’s the dose for which we
have known survival benefit in the
metastatic setting. The bevacizumab is
administered at the 15-mg/kg dosing
every three weeks starting with the
first cycle of chemotherapy and then
continuing for one year.
We are limiting patients with Stage
IB disease to those whose tumors are
four centimeters or larger. We know
from subset analyses of the larger adjuvant
trials that patients with Stage IB
disease don’t seem to benefit overall.
The CALGB IB trial was statistically
negative overall, but those whose tumors
were four centimeters or larger did show
a survival benefit. That’s why we came up
with the 4-cm cutoff.
At this point we’re not limiting to any
specific non-small cell histology. We’re
also not excluding patients receiving
anticoagulation.
Based on the safety data that have
emerged in colorectal cancer — and now
hints that have emerged in the AVAiL
study — patients who have had any sort
of stroke or transient ischemic attack
are excluded. Patients who have had any
other arterial thrombotic events within
six months — such as myocardial infarction
— are also excluded.
Click on the image to enlarge
Select Publications
|
