| |
Treatment of Metastatic Non-Small Cell Lung Cancer |
Lung Cancer Update 2007 (3)
DR JOHNSON: In terms of our algorithm
for the management of metastatic
disease in the clinical setting for
patients who are not in the EGFR-enriched
populations, we follow the
Eastern Cooperative Oncology Group
(ECOG) algorithm. For patients
with adenocarcinoma without brain
metastasis, serious cardiovascular or
cerebrovascular problems or clotting, we
recommend paclitaxel, carboplatin and
bevacizumab. For patients with squamous
cell carcinoma, brain metastasis or
hemoptysis, we administer paclitaxel and
carboplatin without bevacizumab. We
try to utilize the same drugs off study
as we do on study. For patients with a
number of comorbidities, we administer
a single agent such as vinorelbine.
For patients treated with paclitaxel,
carboplatin and bevacizumab, side effects
we see include hypertension and an
increased risk of clotting, bleeding and
proteinuria, which are all manageable.
We also see an increased risk of deep
venous thrombosis and pulmonary
emboli.
For patients in second-line therapy off
study who have been treated with two
agents — most commonly carboplatin/paclitaxel in our setting — and have a
good response and go off therapy for an
extended period, we’ll commonly go to
docetaxel as second-line therapy. For a
patient who shows a mediocre response
to initial chemotherapy, we will generally
use erlotinib as the second agent. We
often use pemetrexed as the third-line
agent. For almost everybody off study,
we use one of the three approved agents
for second-line treatment — pemetrexed,
docetaxel or erlotinib.
Click on the image to enlarge
Lung Cancer Update 2007 (3)
DR WAKELEE: In the setting of first-line
metastatic disease, I believe we’re still left with a platinum doublet,
potentially even a nonplatinum doublet,
as the chemotherapy base. Additionally,
some trials are evaluating carboplatin and
pemetrexed as another platinum doublet.
It’s a reasonable option, and it may be less
toxic than some of the other regimens, but
it’s not better, and you lose an agent that’s
commonly used in the second line. Is
that good or bad? I believe it’s a matter of
order, and I do not believe it’s a huge step
forward; it’s simply a nice alternative.
For my patients in a nonprotocol
setting who are not eligible or for
whom I don’t want to administer
bevacizumab, in the first line I tend to use
carboplatin and gemcitabine. I’ll also use
carboplatin/paclitaxel. It’s between those
two options, and we discuss the toxicity
differences and scheduling differences
with each patient. Occasionally I’ll use a
cisplatin backbone if the patient wants to
be extremely aggressive.
Interview, May 2007
DR ALAN B SANDLER: ECOG-E4599
showed that the addition of
bevacizumab to paclitaxel/carboplatin
in metastatic disease provided a progression-free survival advantage over that
same chemotherapy alone. I believe that
bevacizumab and other VEGF-mediated
agents or VEGF-directed agents have
two distinct mechanisms of action.
First, I believe they have an effect
on the tumor itself. Tumors have leaky
vasculature, and an anti-angiogenic agent
such as bevacizumab appears to help
prune some of the newer vasculature,
diminishing the leakiness and, therefore,
allowing for better drug penetration by
decreasing the interstitial fluid pressure
in the tumor.
Dr Willett at Harvard showed this in patients with rectal cancer, demonstrating
decreased interstitial pressure in
rectal tumors pre- and postbevacizumab.
That’s one effect: providing better chemotherapy
penetration to the tumor.
That would not appear to be as important
in the adjuvant setting, in which
there is no tumor.
The other effect relates to the
concept of eliminating or reducing the
development of new vasculature for
the initial microscopic and then small
tumors, stopping the new blood vessels
from forming and turning the cancer
into a chronic disease. It’s hoped that
mechanism will play a major role in the
setting of early disease.
Click on the image to enlarge
Lung Cancer Update 2007 (4)
DR EDELMAN: In the advanced disease
setting, I’ve held fairly closely to the
ECOG-E4599 eligibility criteria. We
had discussions in which people were
concerned because of the neutropenic
fever or the hemoptysis seen with the
addition of the VEGF inhibitor, but
again, viewing this in the aggregate,
patients did better with bevacizumab.
They lived longer, and so if we have
patients who would have been eligible
for that study, then we do approach them
about the use of bevacizumab.
I have used it pretty much exactly
as it was used on E4599. The only
difference is that I tend to use less
cytotoxic chemotherapy — I use
four cycles, not six, and I base that
on my belief that the evidence is pretty
compelling that pushing the cytotoxics
does not aid you after four courses of
therapy. I’m an advocate of evidence-based
medicine, but here and there one
can do an induction. I could certainly be
criticized, but I believe it’s a reasonable
approach and it’s well tolerated.
Lung Cancer Update 2007 (2)
DR JOAN H SCHILLER: In ECOG-E4599,
our statisticians conducted
an unplanned analysis evaluating
which subpopulations benefited from
bevacizumab and which did not. They
reviewed all the predefined stratification
factors, and none of these resulted in
a difference between whether or not
patients were likely to benefit from
bevacizumab. A difference did appear
between men and women, however,
which is puzzling. Both men and women
derived a benefit from bevacizumab in
terms of response rate and progression-free
survival, but for some reason the men
seemed to have a longer overall survival
rate if they were receiving bevacizumab
compared to chemotherapy alone. For
the women, the survival appeared to be
roughly the same.
Granted, this was a retrospective
analysis, and the women did extremely
well on the control arm. The median
survival for women on the control arm
without bevacizumab was more than 13
months compared to a median survival
of approximately eight and a half months
for the men on the control arm. I don’t
know why women on our control arm
did so well, but that’s one reason why we
didn’t see a benefit.
Click on the image to enlarge
One explanation why women on the
control arm may have done so well is that
they may have received more epidermal
growth factor inhibitors. This study was
conducted when gefitinib and erlotinib
were just coming out. The big news was
that women seemed to benefit more than
men from those drugs, so it’s possible
that the women were more likely to
receive those drugs than the men, and
that’s why the women on the control arm
performed better.
As a practicing oncologist, I have been
treating women with lung cancer with
bevacizumab, based on the fact that this
was not a prospective analysis, and no
difference was seen in the colorectal carcinoma
data evaluating men versus women.
In the breast cancer data, it appears that
women are benefiting from bevacizumab.
Based on those factors, I’ve continued to
treat women. Moving forward, gender
will be a major stratification factor.
Lung Cancer Update 2007 (3)
DR JOHNSON: My experience with the
carboplatin/paclitaxel/bevacizumab regimen is that it does have added toxicity.
We have used anti-angiogenic agents
for approximately five to seven years, so
we have experience with them. The oral
pills that are the VEGF II inhibitors
share some of the same side effects, which
include high blood pressure and increased
risk of clotting, bleeding and proteinuria.
The side effects are manageable,
as with many of the other agents we use.
The hypertension is treatable, and we
handle most of it ourselves.
The risk of clot is real, however. We
see an increased risk of deep venous
thrombosis and pulmonary emboli.
When you apply the algorithm of limiting
this treatment to the adenocarcinomas
with no history of hemoptysis,
you don’t see much of a problem
with hemoptysis and with other risks of
bleeding, although in the randomized
trial it clearly runs around two or three
percent. This is true for both the US
and European trials, even within that
selected population.
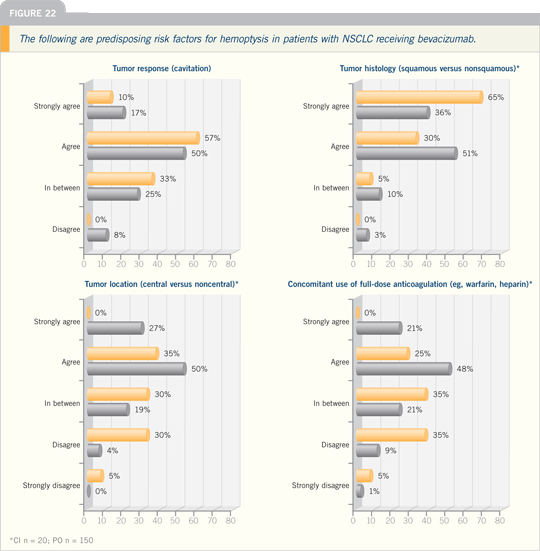
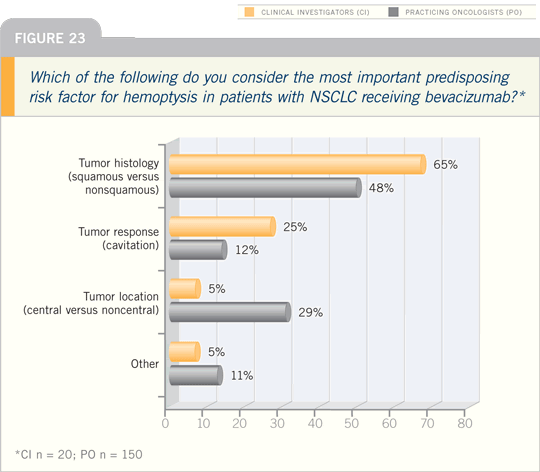
In terms of the patients who have
bleeds, we’ve identified squamous cell
cancer and a history of hemoptysis as
risk factors.
In my experience, one of the differences
with the anti-angiogenic agents, and this
is true of both bevacizumab and the
small-molecule inhibitors of the VEGF
receptors, is that these lesions can cavitate.
It’s different than what we’ve typically
seen with cytotoxic therapy alone.
These spherical lesions hollow out in
the middle and develop a cavity, which
appears to be associated with the development
of hemoptysis.
The assumption is that the antiangiogenic
agents block the blood flow
to the middle of the tumor, it necroses
and you lose some of the structure. The
blood vessels can’t regrow, and they bleed
into it.
In the trials we designed using agents
directed against VEGF, we have not
held therapy if it’s an uncomplicated
cavitation. With any hemoptysis an
oncologist will obviously stop treatment,
but so far we don’t have enough evidence
to stop treatment for a cavitation.
Click on the image to enlarge
Lung Cancer Update 2006 (4)
DR SANDLER: We attempted to define
prognostic variables for pulmonary
hemorrhage in patients who received
bevacizumab. It was a case control study
in which we combined the data sets from
a Phase II study with those from the
ECOG-E4599 study and attempted to
assess a wide range of prognostic variables
to see if one could better define
which group of patients was more at
risk.
We looked at 22 patients with Grade
III or higher pulmonary hemorrhage.
Not surprising with the limited number
of patients, nothing was statistically significant,
but there appeared to be trends
for patients with baseline cavitation in
their tumors and a history of hemoptysis
that predated treatment.
Patients with hemoptysis were not
allowed in the study. In ECOG-E4599,
it was not specifically written into the
study at first, but then one or more
patients entered the study who had
hemoptysis. After the first 60 or so
patients, it was put in specifically as an
exclusion criterion.
In our study, we had an independent
radiology group examine all the individual
CT scans, and tumor size and
location did not seem to correlate with
pulmonary hemorrhage. We saw a hint
that endobronchial disease might be an
issue, although that was not statistically
significant and it is a very difficult interpretation
on a CT scan, and the results
were inconsistent across all the CT scans
and techniques.
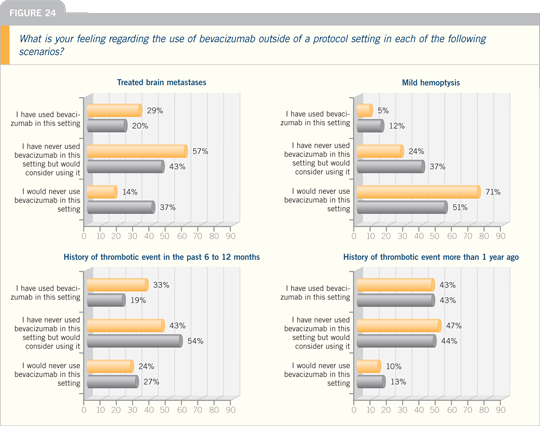
Lung Cancer Update Think Tank 2006
DR LYNCH: We’re participating in a trial
to answer the question regarding the use
of bevacizumab in patients with treated
brain metastases.
Patients have their brain lesions radiated
first, and then they receive chemotherapy
with bevacizumab. Because of
the restrictions in eligibility for ECOG-E4599,
which did not allow patients with
CNS metastases, I believe we have to
follow an evidence-based approach, and I
have not been using bevacizumab in this
setting outside of a protocol.
DR MILLER: We may be amending the
current bevacizumab clinical trials to
allow patients with previously radiated
brain metastases. These contraindications
to anti-VEGF therapy have relative degrees. Certainly squamous histology
and hemoptysis are much more powerful
contraindications. This drug is very active
in patients with glioblastoma multiforme
— huge tumors with lots of edema —
and we’re undertaking approval strategy
trials for those patients. We usually
obtain the blessing of a neurologist to use
bevacizumab in treated brain metastases,
but we certainly have done it.
DR ROY S HERBST: I would wait until
more data are available to use bevacizumab
in patients with CNS lesions,
which I expect will be soon. One trial,
called PASSPORT, will determine if
you can use chemotherapy with bevacizumab
for patients with previously
treated brain metastases.
Interview, July 2007
DR HANNA: At ASCO 2007, Christian
Manegold presented a randomized Phase
III study called the AVAiL trial. Patients
with metastatic disease received cisplatin/gemcitabine with a placebo, bevacizumab
at 7.5 mg/kg or bevacizumab
at 15 mg/kg. The primary endpoint was
originally overall survival, but it was
amended to progression-free survival.
Both the 7.5 mg/kg and the
15 mg/kg arms had a statistically significant
improvement in progression-free
survival. Although they were not
meant to be compared to one another,
the two bevacizumab arms appeared to
improve the progression-free survival by
just about the same amount.
The toxicity profiles of the two dose
levels were very similar. The 15 mg/kg
dose had a little more bleeding than both
the control arm and the 7.5 mg/kg arm.
It also had a higher rate of Grade III and
IV hypertension. There may have been
some slight toxicity disadvantages, with
no apparent efficacy advantages with the
higher dose.
The incidence of bleeding was low
overall. The fear was that the cisplatin/gemcitabine chemotherapy would cause
more thrombocytopenia, and when combined
with bevacizumab, it might result
in some higher-risk bleeding.
The rate of fatal pulmonary hemorrhage
was one percent or less on all three
arms — that wasn’t the concern. It was
other Grade III and IV hemorrhages, which trended a little worse for the 15
mg/kg arm. They didn’t assign a p-value,
so I am not sure whether or not it was
statistically different.
Lung Cancer Update 2007 (3)
DR SOCINSKI: As a purist, I’d point out
that the AVAiL trial wasn’t designed to
address the dose question.
The way I interpret AVAiL is that
it’s a second positive trial evaluating the
use of bevacizumab in combination with
chemotherapy — in this case, cisplatin/gemcitabine. The regimen appears to be
safe, and both the 7.5-mg/kg and the
15-mg/kg doses improved the primary
endpoint of progression-free survival.
No survival data were presented.
The 7.5-mg/kg dose did not appear
to be less toxic, and I have continued
to use 15 mg/kg, based on the survival
results from ECOG-E4599. I would bet
that at least by ASCO 2008, we will see
some survival data from the AVAiL trial,
and perhaps that will change our minds
about the dosing. For right now, in the
absence of survival data in that trial, I’ve
continued administering the 15-mg/kg
dose.
Lung Cancer Update 2007 (3)
DR WAKELEE: In the United States,
carboplatin/paclitaxel with bevacizumab
is approved. Given the AVAiL data,
gemcitabine/cisplatin with bevacizumab
would certainly be a reasonable approach
now.
We’re conducting an ongoing trial
with carboplatin/gemcitabine/bevacizumab.
I wouldn’t say that regimen is
“ready for prime time” — not until we
have the toxicity data, given the increased
thrombocytopenia and neutropenia with
carboplatin/gemcitabine. Substituting
docetaxel for paclitaxel is also reasonable
because we don’t have any toxicity differences
that would be of concern.
AVAiL was a European study
of gemcitabine and cisplatin with or
without bevacizumab. It evaluated two
doses of bevacizumab: 7.5 mg/kg or
15 mg/kg. The 15-mg/kg dose was
the dose used in the ECOG-E4599
carboplatin/paclitaxel study.
AVAiL demonstrated a statistically
significant improvement in progression-free
survival — not a big difference, but a
real difference statistically — with both
the 7.5-mg/kg and the 15-mg/kg doses.
The trial wasn’t powered to compare
15 mg/kg to 7.5 mg/kg — only both of
those doses to placebo. Overall survival
data weren’t mature yet.
The big question is whether we
can get away with using 7.5 mg/kg of
bevacizumab. I’m cautious still. We don’t
have the survival data yet. We have no real
way of evaluating any difference between
15 mg/kg and 7.5 mg/kg, even if we
could do it statistically. I don’t believe it’s
wrong to consider using 7.5 mg/kg, but
I’m not ready to make the change in my
practice. Certainly we won’t be making
a change in the ECOG-E1505 adjuvant
trial, in which we’re still using the
15-mg/kg dose every three weeks.
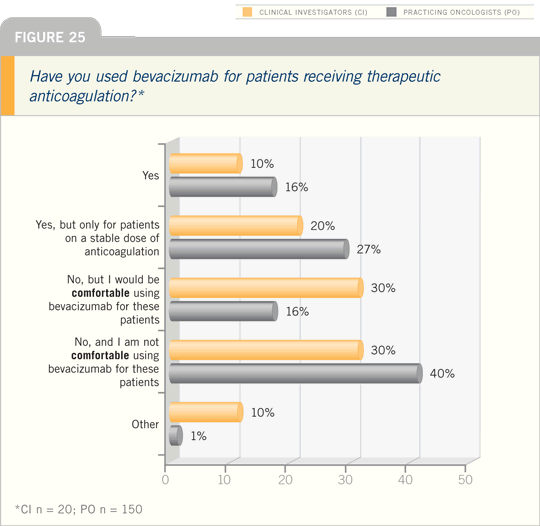
Of note, the bleeding risk in the
AVAiL trial was lower than expected.
They didn’t observe any significant CNS
hemorrhages, which is an issue that had
been raised in ECOG-E4599.
Approximately nine percent of
patients on the trial were on therapeutic
anticoagulation. This was an exclusion
criterion for people going on, but once
they were on the trial, if they ended up
needing anticoagulation therapy, they
were able to stay on the study. There
was no increased bleeding for that group
either.
Click on the image to enlarge
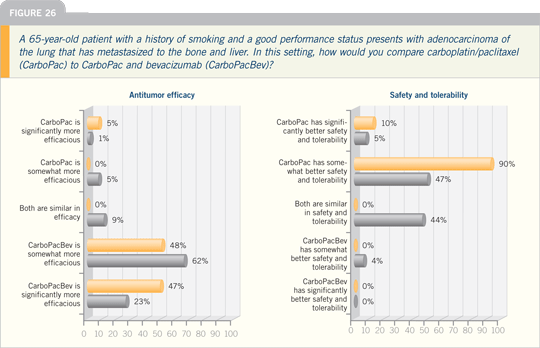
Lung Cancer Update 2007 (3)
DR SOCINSKI: The question that I am
asked most frequently by practicing
oncologists about metastatic disease is
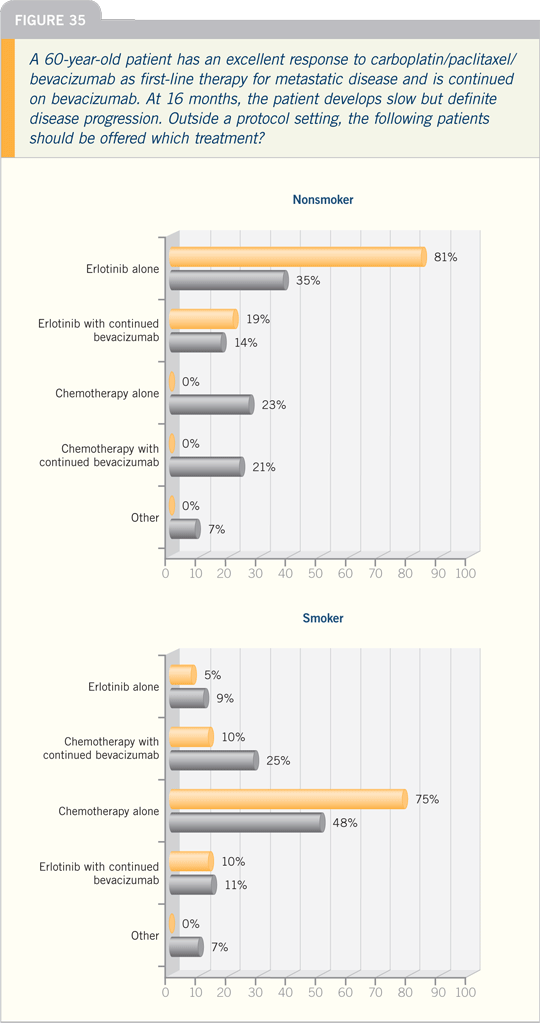
regarding how to approach never smokers.
The never smokers represent approximately
10 percent of the population. In
my experience, if you use the cutoff of
10 to 15 pack years, the oligosmokers
comprise approximately another 10
percent. So one in five patients with
lung cancer fall into this category. That’s
not insignificant when you consider the
number of patients with lung cancer.
The one observation I am convinced
of in that population is that anti-EGFR
therapy seems to be important. The question I struggle with regarding the
never smokers is that many of them are
eligible for bevacizumab. What do you
do in that setting? Are they candidates
for erlotinib or bevacizumab? What’s the
role of chemotherapy?
One option is to treat these patients
with chemotherapy and bevacizumab
and then, as we continue the bevacizumab,
perhaps add erlotinib. We have
a lot of safety information, and I don’t
believe we’re going to harm patients with
that approach.
If patients are not bevacizumab candidates
— let’s say they have brain metastases
— then the question is, should we
use chemotherapy followed immediately
by a maintenance strategy with erlotinib
or chemotherapy with erlotinib or erlotinib
alone?
We currently have the CALGB-30406
trial that randomly assigns these patients
to erlotinib alone versus carboplatin/paclitaxel with erlotinib. It is exploring two
of the three possibilities. You might argue
that we should have used four cycles of chemotherapy followed immediately by erlotinib
or chemotherapy alone as a control
arm, but there are only so many questions
you can ask in a randomized Phase II trial
to sort out these issues.
Lung Cancer Update Think Tank 2007
DR MILLER: I tend to use erlotinib more
either in the first- or third-line setting.
I don’t have a huge second-line cohort.
I’m driven by knowing either the EGFR
mutation status or the clinical factors to
incorporate erlotinib into therapy early
on. If someone has a favorable profile
— a 75 percent positive predictive value
for a response to erlotinib, for example
— those patients live for a long time,
and it’s only a matter of time until we
establish a survival benefit for patients
with EGFR mutations, treated in that
fashion, rather than with chemotherapy.
We need the trials to be conducted, and
they’re ongoing.
Lung Cancer Update 2007 (3)
DR JOHNSON: We believe it’s important to design a clinical trial to ask the question
whether patients who have EGFR
mutation-positive disease will perform
better with front-line EGFR-directed
therapy like gefitinib or erlotinib. We
believe that for two reasons. One is that
time to progression, at least with the
axon 19 deletion mutants, is approximately
one to one and a half years, which
is two to three times longer than with
conventional chemotherapy. In addition,
the survival with that group in the retrospective
studies is approximately three
years, and that’s in comparison to 10 to
12 months with other protocol groups.
It’s important to set up the clinical
trials to show that that’s the case.
However, the process for obtaining the
gene sequence is not easy. You need
to have 300 to 500 tumor cells, and
they have to undergo DNA sequencing,
which is currently the approved test. The
results of that test take approximately
two weeks. People come to the conclusion
that we haven’t seen the definitive
evidence that mutation testing should be incorporated in practice.
I believe we need to take the steps
to show that is the case. Some of us
have been able to integrate it into our
practices, and we use it for making decisions
regarding whether patients should
receive initial treatment with gefitinib or
erlotinib.
Click on the image to enlarge
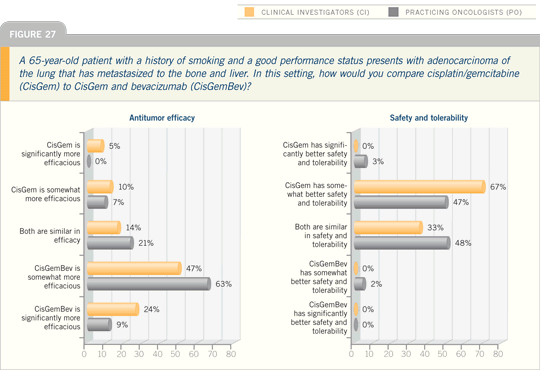
Lung Cancer Update 2007 (4)
DR WALTER J CURRAN JR: Erlotinib
is generally better tolerated, especially
compared to doublet-based chemotherapy.
If it provides the same palliation
and arrest of symptoms you might see
with doublet chemotherapy at the start
and you have a never smoker or an
oligosmoker in a low PS state at diagnosis,
perhaps a bit on the elderly side, I
would like to have data to support erlotinib
as initial treatment for that patient.
We don’t have the data, but I’m hoping
to see it because erlotinib is an option
that many patients and families would
prefer.
Lung Cancer Update Think Tank 2007
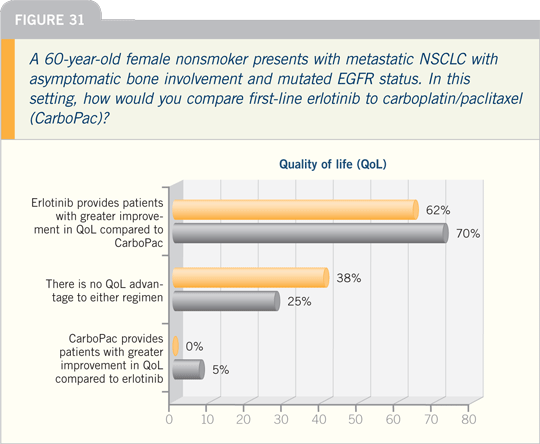
DR KIM: We don’t conduct EGFR mutational
testing on everyone who walks
through the door. But when we see clinical
factors predictive of a response to erlotinib,
mostly the never smokers or those
with adeno-bronchoalveolar features, I
offer them the standard option, which
would be chemotherapy/bevacizumab.
The second aspect would be to consider
the nonstandard therapy, erlotinib.
I presented those options to two different
patients on the same day. One
was a 60-year-old female who was a light
smoker, five pack years, in her twenties,
and the other one was 30 to 35 years old
and a never smoker. Both elected the
first-line erlotinib option.
Interview, July 2007
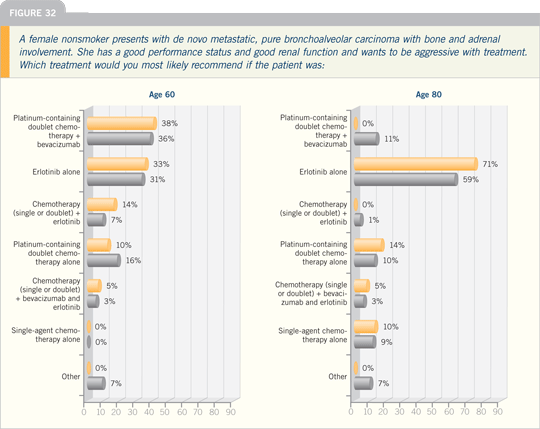
DR HANNA: In approaching a patient
with metastatic disease in the clinical
setting in the first- and second-line situation,
the most important questions are:
What is the performance status of the patient? What are the comorbidities? Is
the patient losing weight? What’s his or
her appetite like? If a patient is PS 3 or 4,
clearly, the right thing is best supportive
care.
If the patient is PS 2, but in addition
is having significant loss of appetite,
loss of weight and comorbidities, then
I believe the appropriate thing for that
patient is best supportive care, unless the
patient is a never smoker. Then I would
consider single-agent erlotinib.
For patients who are PS 0 or 1 and don’t
have contraindications to chemotherapy,
I believe a platinum-based two-drug
regimen is standard. For patients who
are bevacizumab eligible, the addition
of bevacizumab is reasonable. That
would include patients who don’t have
brain metastases, squamous histology,
a history of hemoptysis or uncontrolled
hypertension. I treat those
patients initially with two courses of
chemotherapy and repeat the CT scan. If
they appear to be experiencing a clinical
benefit, I administer four courses of chemotherapy. Because the ECOG-E4599
study continued patients on bevacizumab,
I administer that in maintenance until
the time of progression.
Click on the image to enlarge
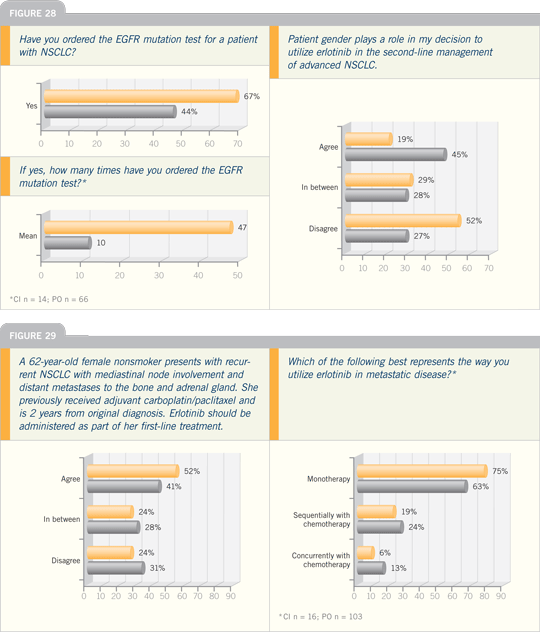
Click on the image to enlarge
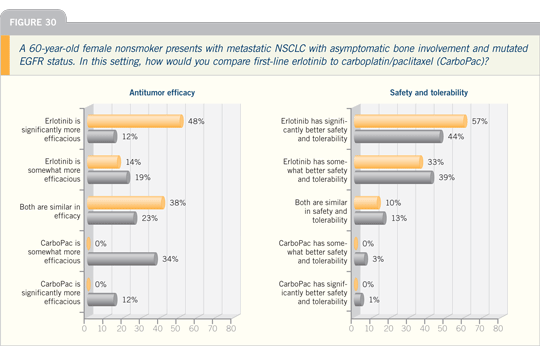
Lung Cancer Update 2007 (4)
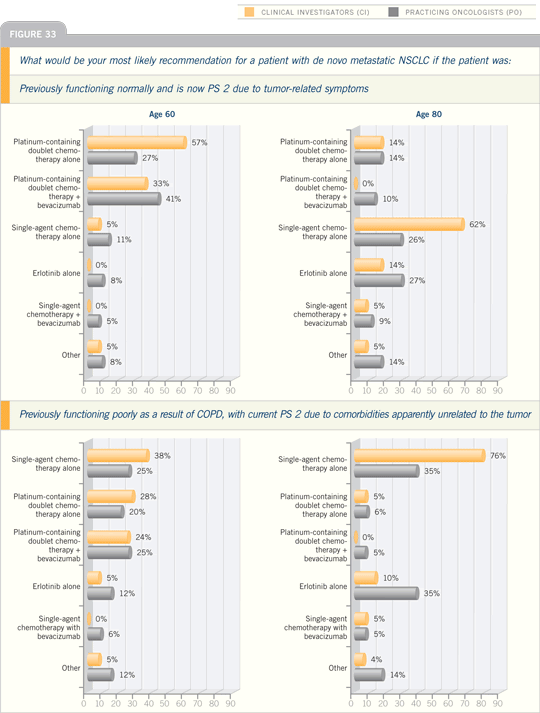
DR EDELMAN: I approach PS 2 patients
by administering a dose-attenuated,
platinum-based regimen. I have found
the carboplatin/gemcitabine regimen
to be extremely well tolerated. We’ve
used that, either the two drugs by
themselves or sequentially, followed by
weekly paclitaxel.
This is also well tolerated. I believe
there’s a fair amount of evidence that
says that those who doubt the role of a
platinum agent in PS 2 patients should
consider repenting: 1) the study that was
presented by Obasaju of carboplatin/gemcitabine versus gemcitabine, which
showed similar results for the overall
population and the PS 2 preplanned
subanalysis, and 2) the CALGB study
that evaluated carboplatin/paclitaxel versus paclitaxel, which showed that if
anything, the PS 2 patients probably see
the most dramatic degree of benefit from
the addition of a platinum agent.
Why is that, and why do we have this
significant split? I believe it’s because
PS 2 is a heterogeneous group. There
are three groups of patients who end up
what we call, in our simplistic way, PS
2. There are those whose performance
status has decreased as a consequence
of their disease. We all see patients who
come in, and they have their families
who say, “This guy was working, doing
manual labor four weeks ago,” and they
say, “Yeah, now it’s a pain to get up and
walk around.” Reasonably, they’re still
walking around, doing normal activities,
but they’re too fatigued or they’re weak
and they’ve lost weight. That’s one group.
Those are the disease-result PS 2s.
You have a second group that’s been PS
2 for 20 years. They have comorbidities.
They never get out of bed to begin with.
That’s another bunch, and then you also
have frail individuals. We all know this
type of patient, the little old lady who
looks as if she’s going to get blown away
in the next wind storm, and they have
poor muscle mass — they’re doing fine
until suddenly they’re not. I think those
latter two groups, the ones who have
significant comorbidities and the frail
patients, tend not to do well when they
receive a two-drug regimen because, for
one reason or another, it aggravates their
preexisting comorbidity.
Click on the image to enlarge
Lung Cancer Update 2007 (3)
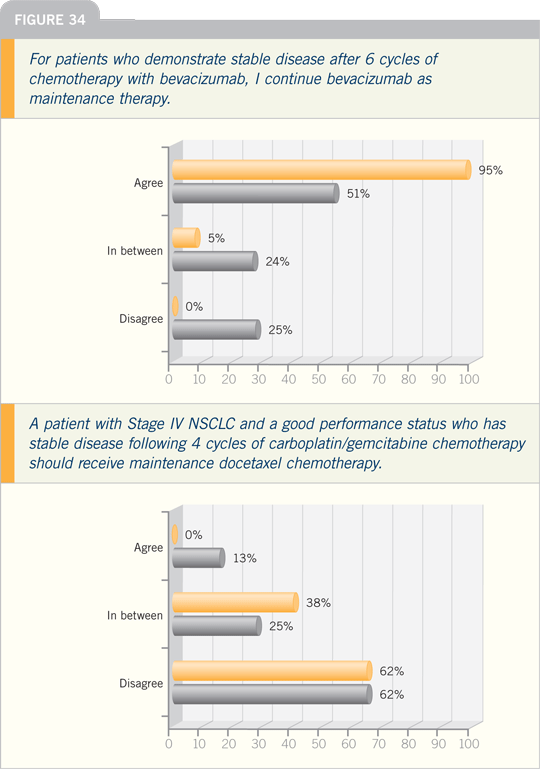
DR SOCINSKI: There was a very interesting
trial presented at ASCO this year
designed to evaluate the administration
of immediate maintenance docetaxel
following four cycles of first-line doublet
chemotherapy versus second-line
docetaxel per the standard approach,
which is to wait until time of progression.
Their ability to deliver second-line
therapy was much higher in the immediate
group than in the delayed group.
We know if you let the natural history
of this disease play out, things happen
and patients who are good candidates
for treatment become marginal- or notreatment
candidates, based on declining
performance status and disease-related
symptoms.
Click on the image to enlarge
We know that second-line therapy
works, but it can only be effective if
you can administer it to the patient. So,
post-ASCO 2007, this raised the question
in my mind: How do you follow
patients after four to six cycles of first-line
chemotherapy? What triggers you
to institute second-line therapy? I do
not consider myself an overtester so I do
not perform a lot of x-rays and CT scans
while following patients, but I do see
them every four to six weeks. I can tell a
lot just by their appetite, pain level and
chest x-ray. But I tend to think that obviously,
you’re not going to benefit patients
with second-line therapy if they end up
not being good treatment candidates.
This trial also suggested that the time
to disease progression was improved with
immediate docetaxel rather than waiting
until disease progression to institute
second-line chemotherapy. There was a
trend toward improved survival in this
setting. We know that second-line therapy
improves survival, but if you don’t
receive it, you’re not going to live longer.
Lung Cancer Update 2007 (1)
DR RONALD B NATALE: A major study
was presented by Roy Herbst at ASCO
2006 evaluating the combination of bevacizumab
and erlotinib in the second-line
setting. This was an unselected group of
patients, and the objective response rate
was close to 25 percent, which is considerably
higher than the 10 percent or so
objective response rate one would expect
with erlotinib alone.
That was encouraging and has led
to a definitive randomized Phase III
trial in which I am participating, and in
fact, I am one of the major accruers to
the BETA (bevacizumab and Tarceva®)
study. This is a randomized trial in the
second-line setting in which all patients
receive erlotinib and then either placebo
or bevacizumab every three weeks.
That study will answer the question as
to whether the combination confers a
benefit.
Lung Cancer Update 2007 (3)
DR SOCINSKI: The attractiveness of
combining erlotinib and bevacizumab
is that they target two new, validated
pathways. Each agent has been shown
to improve survival. It’s a novel targeted
approach that breaks away from some
of the traditional toxicities we have with
regular chemotherapy. It makes biologic
sense to combine them.
The initial data we had from MD
Anderson and Vanderbilt were encouraging,
and a randomized Phase II trial
suggested that bevacizumab added to
chemotherapy or erlotinib was better
than chemotherapy alone. It also suggested
that the combination of erlotinib
and bevacizumab appeared as good, with
less toxicity, than the chemotherapy/bevacizumab arm. The bevacizumab/erlotinib combination opens up the possibility
that some patients may be better
served with a noncytotoxic approach.
I believe the jury is still out on that
issue. Phase III trials are ongoing that
will answer the question about combination
bevacizumab/erlotinib. We also
have to remember that we may be able to
identify with various biomarkers patients who, at least from the erlotinib point of
view, may be the best candidates for that
approach.
Select Publications
|
