| |
Systemic Therapy for Metastatic Disease |
Miami Breast Cancer Conference Tumor
Panel Discussion
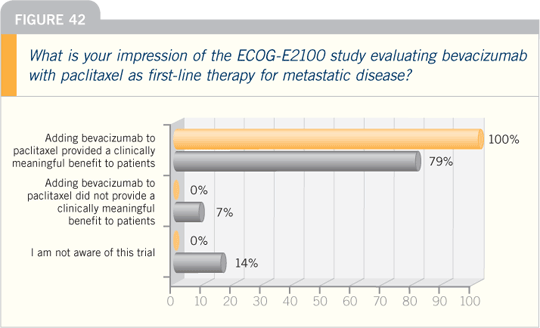
DR BURSTEIN: The exciting thing about
ECOG-E2100 was it really established
a principle that anti-angiogenic therapy
can be effective in advanced breast cancer.
We had the results from the previous
trial of anthracycline- and taxane-treated
patients who were randomly assigned
to capecitabine with or without bevacizumab,
and in that study it was hard to
see much significant clinical benefit.
It was always hard to square that
result with the data that were seen in
colorectal cancer. It’s not clear that one
is such a vascular-driven tumor and the
other would not be. So, from a conceptual
point of view, the ECOG-E2100
really opens up a whole new area for us to
try and exploit to help take better care of
cancer patients, and that’s why I think it’s
a very compelling study. It really gives us
something tremendous to build upon.
Meet The Professors 2005 (3)
DR O’SHAUGNESSY: It’s not too surprising
that E2100 was a positive trial because
as a single agent, it has activity. It also
had activity in the capecitabine randomized
Phase III trial. That was a late-line
population, and it’s difficult to change the
median of anything when only a small
percentage of patients benefited.
I guess the number one thing I want
to see are some interesting exploratory
subset analyses. For example, is the benefit
of bevacizumab going to be largely
seen in the higher-grade tumors, such
as ER-negative, PR-negative or perhaps
HER2-positive tumors?
What about indolent disease? You
might have patients with some indolent
biology, so I need to see the data.
However, this sounds like a real advance
for select patients for whom you believe it
will be safe to administer bevacizumab.
Breast Cancer Update 2006 (4)
DR KATHY D MILLER: In the design of
ECOG-E2100, we allowed patients who
had received a taxane-containing adjuvant
regimen to enroll as long as their disease-free
interval was at least 12 months. We
did that for pragmatic reasons because the
taxanes were being used more frequently
in the adjuvant setting. We thought it
would be reasonable to consider re-treating
those patients if their disease-free
interval was at least a year.
Approximately 18 percent of our
patients had received a taxane-containing
regimen. Their hazard ratio was 0.38,
which was the best hazard ratio of all of
the clinically based subsets. For those
patients, that translated into an improvement
not from six to 11 months but from
four to just more than 12 months in
median progression-free survival.
We talked about whether we should
have a crossover in ECOG-E2100, and
we decided not to for a couple of practical
reasons. One was that it would have
made the trial a lot more complicated
and expensive. Also, our primary endpoint
was progression-free survival, so
having a crossover would not have contributed
to our primary endpoint.
Breast Cancer Update 2005 (7)
DR WINER: I believe the results of
ECOG-E2100 are impressive enough
that, in the absence of a contraindication
to bevacizumab, I would now use it in a
first-line setting, optimally in combination
with paclitaxel as administered in
the study.
I doubt that the interaction is specific
between paclitaxel and bevacizumab,
although I’m well aware that when given
with capecitabine in more advanced disease,
bevacizumab seemed to be less
active. I believe that’s probably related to
the setting rather than the drug.
Breast Cancer Update 2005 (6)
DR SLEDGE: As a result of the previous
toxicity seen in the lung cancer trial, we
had very stringent criteria for discontinuing
E2100 if we saw an excess number
of patients developing Grade IV hypertension
or bleeding. When the trial was
initiated, the National Cancer Institute
had significant concerns about patient
safety as a result of the initial experience
with bevacizumab in lung cancer.
Fortunately, early analyses demonstrated
that was not an issue in breast
cancer. The side effects were relatively
minimal. Predominantly, we saw mild
to moderate increases in blood pressure,
which is readily handled from a clinical
standpoint. Of course, we’ll have to
be careful with the hypertension as we
move bevacizumab into the adjuvant setting.
We also saw a low incidence of serious bleeding. Overall, bevacizumab was
a nontoxic addition to chemotherapy.
Breast Cancer Update 2006 (4)
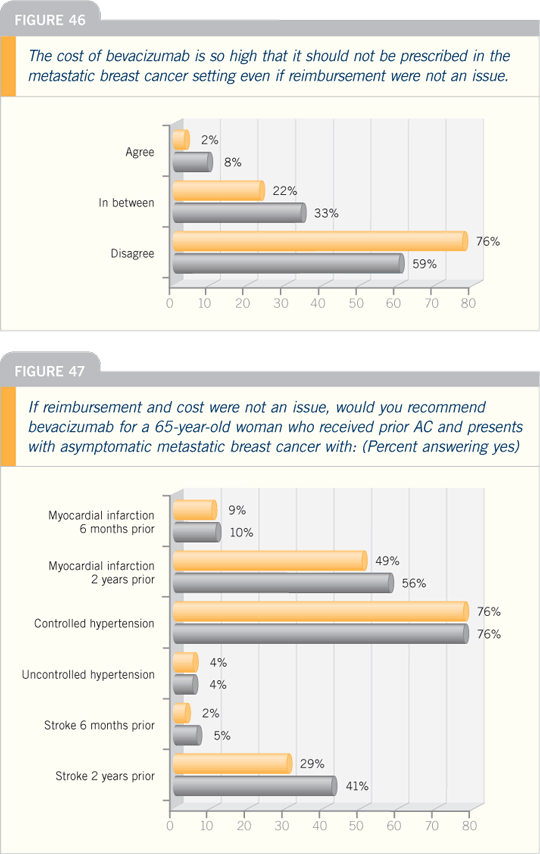
DR MILLER: Adding bevacizumab
increases the risk of arterial thrombotic
events, although to a very modest degree.
We know a little about the risk factors
in that the risk seems to be preferentially
borne out in patients who are older than
age 65 or those who have had previous
arterial thrombotic events, particularly
MI, TIA or stroke.
No reports associate cardiomyopathy
or congestive heart failure with bevacizumab
in any of the trials that either
did not use concurrent anthracyclines or
were in patient populations who would
not have been previously treated with
anthracyclines. So this is an issue specific
to patients with breast cancer, sarcoma
or leukemia, for which anthracyclines
are used.
In the randomized bevacizumab/capecitabine trial, two patients had congestive
heart failure or cardiomyopathy
in the capecitabine-alone group compared
to seven in the capecitabine with
bevacizumab group. That sounds like an
increase, but the overall event rate was
so low that, statistically, those numbers
were not different.
In ECOG-E2100, we didn’t see any
sign of congestive heart failure when
comparing the two groups. In Sandy
Swain’s 21-patient experience, which is the only breast cancer trial that has used
an anthracycline and bevacizumab concurrently,
none of the patients had clinical
congestive heart failure, but two of
them showed a decrease in their ejection
fraction to less than 40 percent.
Click image below to enlarge
Breast Cancer Update — Think Tank
Issue 1, 2006
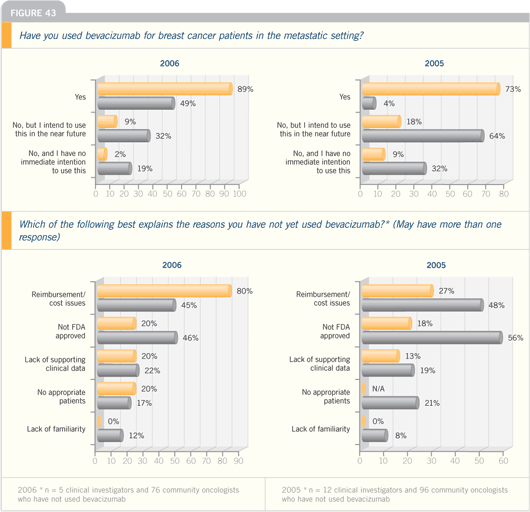
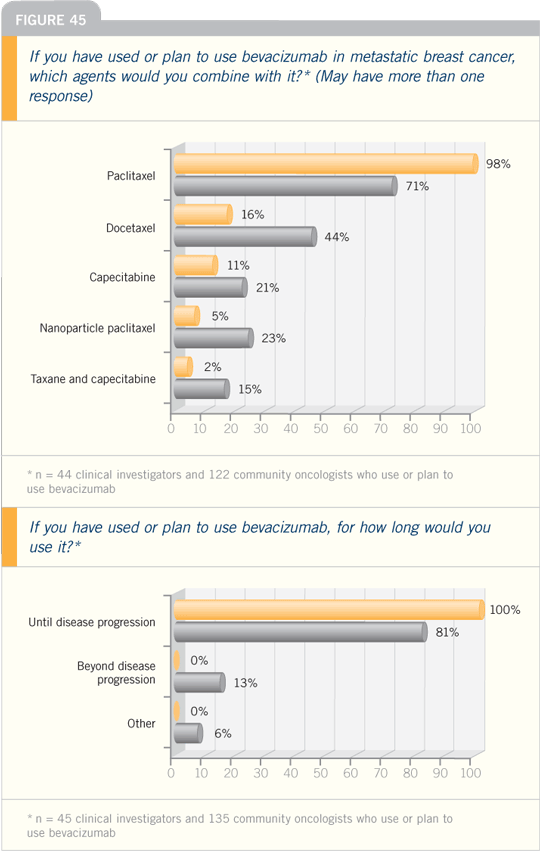
DR WINER: Three issues have led people
to be less enthusiastic about bevacizumab
use in first-line metastatic breast cancer.
One is that the E2100 data apply to a large
subset of patients. They would be happier
if it were targeted to a smaller specific
subset of patients. The second is that they
are less enthusiastic and unsure of what
to do with the capecitabine trial. And the
third and very real issue is the cost.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR SLEDGE: From a quality-of-life
standpoint, those of us who have used
bevacizumab have found it an incredibly
easy drug for patients. The toxicity
is truly trivial compared to every single
chemotherapeutic agent in the therapeutic
armamentarium. So it’s not had any
major negative effect on any significant
percentage of patients in terms of quality
of life and increase in toxicity.
However, these are the issues regarding
bevacizumab extension: First, safety
of prolonged exposure to bevacizumab;
second, response to second-line combination
therapy with bevacizumab; third,
issues surrounding resistance to antiangiogenic
therapy; and then, finally, the
cost of therapy.
Breast Cancer Update 2006 (4)
DR MILLER: One of the trials that we
activated shortly after we had the results
from ECOG-E2100 was a Phase II
trial known as XCaliBr, which uses the
capecitabine/bevacizumab combination
from the earlier Phase III trial but as
first-line therapy for patients with metastatic
disease. It’s essentially the ECOG-E2100
patient population using the regimen from the capecitabine/bevacizumab
trial. We thought that was a reasonable
trial because we had ample safety data
with the combination, and we knew
that adding bevacizumab to capecitabine
improved the response rates.
It potentially will provide patients
in that first-line chemotherapy setting
another option and one that would be
oral and wouldn’t cause alopecia, if we
see similar response rates and progression-free survival in a decent-sized Phase
II study.
Our trial with refractory patients
found a doubling of response rates. We
have data that strongly suggest this
would be active. What we don’t know
is whether we’ll have the same response
rate and progression-free survival as with
the paclitaxel-based regimen. I believe
that would be an important piece of data
clinically to allow people greater flexibility
in their first-line regimen of chemotherapy
with bevacizumab.
Breast Cancer Update 2006 (3)
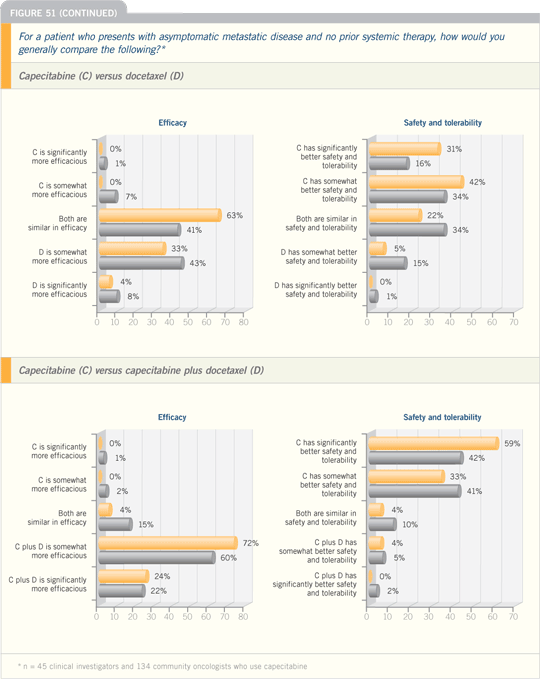
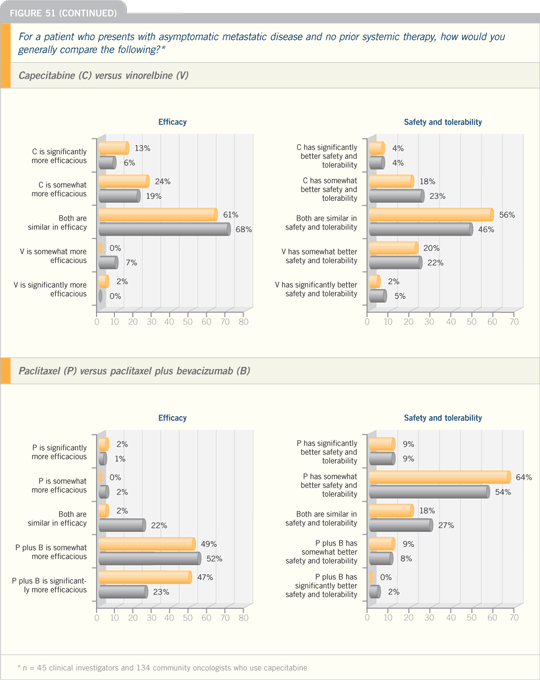
DR GRADISHAR: I still believe that
capecitabine is a good up-front agent
to use in metastatic disease for many
patients, and that hasn’t changed with
the bevacizumab data. However, the
data that emerge from the XCaliBr
study may provide justification for using
capecitabine with bevacizumab, assuming
the data are positive and comparable
to what we saw in the E2100 study.
Capecitabine is comparable to our
most active chemotherapy drugs, but
I don’t view any drug as the best agent
in a particular situation. I would use
capecitabine for patients with minimal
visceral disease such as small liver metastases,
but docetaxel or nanoparticle albumin-
bound (nab) paclitaxel would be
fine as well. It’s a judgment call that you
make with each patient depending on
her preferences.
Miller KD et al. Randomized phase III trial
of capecitabine compared with bevacizumab
plus capecitabine in patients with previously
treated metastatic breast cancer. J Clin
Oncol 2005;23(4):792-9.
The addition of bevacizumab to
capecitabine clearly increased response rates, whether assessed by the IRF or
the investigators, without significantly
adding to the overall toxicity of the treatment
regimen.
Despite improvement in ORR, the
duration of the responses was short with
respect to PFS, and the proportion of
long-term responders was similar in the
two groups.
Burstein HJ et al. Metronomic chemotherapy
with and without bevacizumab for advanced
breast cancer: A randomized phase II study.
San Antonio Breast Cancer Symposium
2005;Abstract 4.
Metronomic chemotherapy administered
to this patient population using
low dose oral cyclophosphamide and
methotrexate had minimal clinical activity
by itself. In combination with metronomic
chemotherapy and bevacizumab,
there was a higher clinical activity rate
noted in women with advanced breast
cancer.
We believe the combination therapy is
reasonably well tolerated and lacks many
of the acute side effects of chemotherapy.
We are in the process of performing
correlative studies of VEGF levels
and circulating endothelial cells to both
understand the mechanism of action of
these treatments and to try and identify
patients who might selectively benefit,
and we believe that further investigation
of this treatment option is warranted.
To that end, we have activated a study
at Dana-Farber, Indiana University, and
UCSF, in which patients who have residual
invasive breast cancer after receiving
neoadjuvant chemotherapy will be
assigned to treatment cohorts where
they will receive one year of bevacizumab
or one year of bevacizumab with six
months of metronomic chemotherapy in
a group of women who by some of the
definition have resistance to traditional
chemotherapy.
Breast Cancer Update 2005 (8)
DR VICENTE VALERO: There are two
combination regimens that have proved
to be superior to single-agent taxane
therapy for metastatic disease. One is
gemcitabine with paclitaxel, which was
compared to paclitaxel alone. The data
were presented at ASCO, showing an
improvement in time to progression and
preliminary evidence of an increase in
overall survival.
The other study compared docetaxel
with capecitabine to docetaxel alone and
also showed a time to progression and
overall survival advantage.
Based on the evidence, both of these
combinations are reasonable for first-line
chemotherapy of metastatic disease.
However, in some patients, sequential
chemotherapy is our preference.
I tend to use more sequential single-agent
chemotherapy, but I believe the
role of combination chemotherapy in
some instances is well documented by
the two studies I just mentioned.
For women who have symptomatic
breast cancer with visceral involvement,
it is essential to have a response to alleviate
the symptoms and improve their
quality of life. For those patients, despite
the enhancement of the adverse events,
I strongly consider combination chemotherapy.
Eniu A et al. Weekly administration of
docetaxel and paclitaxel in metastatic
or advanced breast cancer. Oncologist
2005;10(9):665-85.
Optimizing the dose and schedule of
taxane therapy to maximize antitumor
activity while maintaining a favorable
toxicity profile remains an important
goal in MBC. Weekly, rather than
the standard every three weeks, dosing of
docetaxel and paclitaxel at lower doses is
one way to provide an efficacious method
of drug delivery while maintaining a
favorable toxicity profile.
Various studies support weekly taxane
dosing as an active regimen in MBC,
even in heavily pretreated, refractory disease
and in elderly patients or those with
poor performance status. Importantly,
this regimen is associated with a low
incidence of severe hematologic toxicities
and acute nonhematologic toxicities.
Jones SE et al. Randomized phase III study
of docetaxel compared with paclitaxel in
metastatic breast cancer. J Clin Oncol
2005;23(24):5542-51.
This is the first clinical trial to compare
directly the taxanes, docetaxel and
paclitaxel, as monotherapy for patients
with advanced breast cancer. Using
US Food and Drug Administration-approved
doses and schedules for each
agent, this phase III study has demonstrated
that docetaxel is superior to
paclitaxel in TTP (5.7 v 3.6 months; P
<.0001), response duration (7.5 v 4.6
months; P <.01), and OS (15.4 v 12.7
months; P <.03).
The overall response rate was also
greater with docetaxel (32% v 25%; P <
.10). The survival advantage for docetaxel
was observed despite the increased incidence
of toxicities leading to dose reductions
and treatment withdrawal, and
the slightly greater use of salvage treatment
in patients randomly assigned to
paclitaxel.
The results of this study are consistent
with those reported for previous
phase III studies of single-agent
docetaxel and paclitaxel.
Ghersi D et al. A systematic
review of taxane-containing regimens for
metastatic breast cancer. Br J Cancer
2005;93(3):293-301.
We compared the results of randomized
trials comparing taxane-containing
chemotherapy regimens with regimens
not containing a taxane in women with
metastatic breast cancer. The specialized
register of the Cochrane Breast Cancer
Group was searched in March 2004.
Eligibility was assessed and data extracted from eligible studies by two reviewers.
Hazard ratios (HR) were derived for
time-to-event outcomes, and a fixed-effect
model was used for meta-analysis.
Tumor response rates were analyzed as
dichotomous variables.
Of 21 eligible trials, 16 had published
some results and 12 data on overall survival.
An estimated 2621 deaths among
3643 women suggest a significant difference
in overall survival in favor of
taxane-containing regimens (HR 0.93,
95% confidence interval (CI) 0.86-1.00,
P=0.05). The treatment effect on survival
was similar if only trials of first-line
chemotherapy were included, although
not statistically significant.
There appeared to be an advantage
for taxanes in time to progression (HR
0.92, 95% CI 0.85-0.99, P=0.02) and
overall response (odds ratio (OR) 1.34,
95% CI 1.18-1.52, P<0.001). There was
significant heterogeneity across the trials
(P<0.001), partly because of the varying
efficacy of the comparator regimens.
Taxane-containing regimens improved
overall survival in women with metastatic
breast cancer. Taxane-containing regimens
are more effective than some, but
not all, nontaxane-containing regimens.
Breast Cancer Update 2006 (3)
DR GRADISHAR: In terms of first-line
taxanes in the metastatic setting, the
data are still more abundant with both
paclitaxel and docetaxel than with nab
paclitaxel, so if basing a decision on the
length of experience, those agents have
been around for a longer time.
However, I see no reason to believe
that nab paclitaxel will prove inferior to
those drugs with more data. I believe nab
paclitaxel will compare favorably, if not
prove to be superior.
When you examine clinical trials that
have evaluated docetaxel or paclitaxel in
similar patient populations with metastatic
disease, the indirect evidence shows
the activity of nab paclitaxel to be comparable
to docetaxel. These agents may
have similar antitumor effects, so one
should consider other factors, including
toxicities, patient convenience and cost.
If nab paclitaxel can offer the same antitumor effect as docetaxel and
paclitaxel along with advantages in terms
of lack of pre-medication and shorter
infusion time, whether or not it would
become the preferred agent is an important
question. When you think of busy
office practices, the throughput of
patients and convenience to patients are
important. An upside to nab paclitaxel
clearly is the shorter infusion time and
the lack of need for pre-medication.
As for the higher acquisition cost of
nab paclitaxel, economic analyses suggest
that some of the downstream expenses
related to administering paclitaxel or
docetaxel — specifically the costs of pre-medications
and antibiotics or growth
factors to manage the neutropenias or
cytopenias — result in a net savings with
the use of nab paclitaxel.
Although we need more information,
I believe we shouldn’t necessarily be put
off by the up-front cost; we should take
into account the whole package of managing
the patient’s treatment.
Smith I. Goals of treatment for patients
with metastatic breast cancer. Semin Oncol
2006;33(1 Suppl 2):2-5.
The key goal in the treatment of metastatic
breast cancer is to prolong survival,
with an emphasis on restricting treatment-related toxicity as much as possible.
Despite the plethora of treatment modalities
available in metastatic breast cancer,
significant survival differences are relatively
uncommon. Symptom relief and
quality of life are other important, clinically
validated measurement instruments.
Symptom relief in particular is not
used as widely used as it could be, in
contrast to lung cancer where it has been
proven clinically informative. Finally,
time to disease progression is an increasingly
used primary endpoint in comparing
treatments for metastatic breast cancer;
this measure includes both patients
who achieve an objective response, and
those whose disease may be stabilized
with treatment.
Special Edition BCU: Proceedings
from Two Medical Oncology Educational
Forums, 2005
DR RAVDIN: Capecitabine has some attractive features. In terms of toxicity
and response, I view it as something
that bridges the gap between hormonal
therapy and intravenous chemotherapy.
Particularly when dosed a bit lower than
the package insert dose, it’s tolerable for
most patients, and they don’t experience
nausea, vomiting or hair loss, almost as if
they were receiving an endocrine agent.
It’s an oral agent — we don’t have to put
in a line — so it’s easier for patients to
accept. I think all those features make it
an attractive agent.
Actually, I’m surprised that it isn’t
more commonly used in the community
because I think it’s one of those agents
that is generally tolerated with repeated
use. With a lot of other agents, patients
begin to get tired when you get in six
cycles.
Breast Cancer Update 2005 (8)
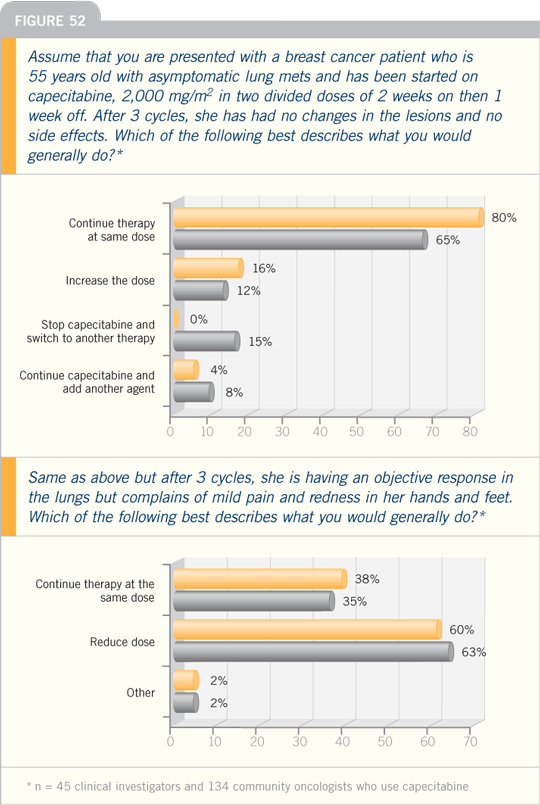
DR VALERO: When using capecitabine in
patients with a good performance status
who are not heavily pretreated, I use
2,000 mg/m2 daily in two divided doses
for 14 of 21 days.
After two cycles of therapy, I will
consider escalating the dose if the patient
has no toxicity. For patients with a poor
performance status, for whom I’m going
to consider capecitabine as a second- or
third-line therapy — patients who are
fragile — I may use 1,750 mg/m2 daily.
We recently published in the Annals
of Oncology about our experience at
MD Anderson with different doses of
capecitabine. We believe that a lower
dose is preferable, although the FDA-approved
dose is 2,500 mg/m2.
I believe this publication confirms
what we do in the clinic. When you have
a Phase II study in several locations, but
you select people out and monitor them
very closely, capecitabine can be administered
at a higher dose.
I could deliver capecitabine at 2,500
mg/m2 daily, but it needs close monitoring
with a patient who is able to follow
closely with her oncologist.
In the clinical trials, as soon as the
patients start to develop early signs of
mucositis, diarrhea or hand-foot syndrome,
capecitabine is stopped immediately.
Then the patient reports to the oncologist
or the research nurse for instructions.
Then you restart the capecitabine,
and you may restart it at a lower dose. So
it needs some close monitoring.
In general, most patients at the end of
the day receive an average dose of around
2,000 mg/m2 daily as a single agent. In
some patients, I also use even lower doses
— 1,500 mg/m2 daily.
The bottom line is that the evidence
that a lower dose is efficacious is just not
there. Our study is one of the first that
provides information, but it was not a
prospective study to assess response and
time to progression in a well-designed
Phase II fashion.
Hennessy BT et al. Lower dose capecitabine
has a more favorable therapeutic index in
metastatic breast cancer: Retrospective
analysis of patients treated at MD Anderson
Cancer Center and a review of capecitabine
toxicity in the literature. Ann Oncol
2005;16(8):1289-96.
We retrospectively reviewed the records
of 141 consecutive patients with metastatic
breast cancer identified from pharmacy
records as receiving capecitabine
outside of a clinical trial between May
1998 and February 1999...
It is apparent that the toxic effects
associated with capecitabine therapy at
2,500 mg/m2/day cause morbidity in a
relatively high proportion of patients,
necessitating frequent dose reduction.
This is consistent with our experience.
Since the most important goal of the
treatment of metastatic breast cancer is
symptom palliation, therapy associated
with considerable morbidity defeats the
purpose. Reduction of the capecitabine
dose has been shown to improve drug
tolerability in most cases.
Moreover, retrospective analysis of
many of the capecitabine trials has found
that dose reduction for adverse events
related to capecitabine did not have an
impact on efficacy of the drug. This is
supported by our data. In our experience,
the mean tolerated dose of capecitabine
is 2,040 mg/m2/day. Thus, it seems
appropriate to use the drug at a lower
starting dose, perhaps 2,000 mg/m2/day
in two divided doses.
Bajetta E et al. Safety and efficacy of
two different doses of capecitabine in the
treatment of advanced breast cancer in older
women. J Clin Oncol 2005;23(10):2155-61.
To the best of our knowledge, this is the
first report specifically dealing with the
use of capecitabine in an elderly population
with breast cancer...
Overall, efficacy of the two starting
doses was similar to that reported in a
previous trial, in which first-line monotherapy
with capecitabine at the dose of
2,500 mg/m2/d resulted in an objective
response rate of 30% in 61 women aged
55 years and older. This study has shown
in a large series that oral capecitabine
is well tolerated and effective in older
women with advanced breast cancer.
Older patients may frequently exhibit
diminished capacity to eliminate drugs,
resulting in unusual sensitivity to standard
dosing regimens.
In light of this, the overall results of
the study suggest that although the dose
groups are small and nonrandomized,
the capecitabine dose of 1,000 mg/m2
twice daily merits consideration as “standard”
for women aged 70 years and older
who are candidates to cytotoxic therapy
for metastatic breast cancer and do not
have severely impaired renal function.
Meet The Professors 2005 (3)
DR O’SHAUGHNESSY: I have been
impressed with the combination of a
taxane and capecitabine in patients with
the bone and liver metastases.
I think the capecitabine dose of 825
mg/m2 BID, 14 days on and seven days
off, is now pretty well established for
combination therapy. I will usually treat
for six or seven cycles with the combination,
stop the taxane and keep going with
capecitabine. The duration of response
that I have seen with some patients has
been remarkable. That is not to say that
you wouldn’t have seen the same if you
had used sequential therapy, but the
duration of responses in a number of
patients is impressive, and it’s gratifying.
In the JCO an Italian group reported
a Phase II first-line metastatic
trial of capecitabine in patients with a
mean age of 73. Patients were started
on capecitabine at 1,250 mg/m2 BID.
Two deaths occurred, so they reduced
the dose to 1,000 mg/m2. The report
included about 40 patients treated with
1,250 mg/m2 and another 43 patients
treated with 1,000 mg/m2. In the trial,
they observed an acceptably low rate of
Grade III toxicities with the lower dose in less than 10 percent of patients. In
both cohorts, the response rate was 35
percent, which is pretty impressive, and
another third of patients treated with
1,000 mg/m2 had stable disease for more
than six months. That is getting remarkably
high.
Click image below to enlarge
Click image below to enlarge
Click image below to enlarge
Meet The Professors 2005 (3)
DR HUDIS: I don’t harbor a firm belief
that the order of single agents matters
as much as people believe it does. For
patients reluctant to receive chemotherapy
for reasons that are largely emotional,
capecitabine allows them to make that
transition and say to themselves, “I’m
not getting intravenous therapy. It’s not
so bad.”
The fact that capecitabine is as active
as many of the intravenous therapies that
we routinely use makes this sort of a silly
point, but it is one that people buy into.
So if I have a patient who is reluctant
to start intravenous therapy, I will look
toward capecitabine.
If I have an older patient, to tell you
the truth, it cuts both ways. The truth
is that capecitabine does bring up compliance
and safety issues related to self-administration.
It’s not crystal clear to
me that it’s always safe for or better for
a person to take medication at home.
Sometimes you have a little more control
over them if you can administer intravenous
therapy and withhold it when you
should.
Breast Cancer Update 2005 (5)
DR NANCY E DAVIDSON: Many times in
metastatic disease we use all of the available
therapies, so what we’re really deciding
on is the order — what to start with.
Many patients make that decision based
on their personal values. I find many
of my older patients are attracted to
capecitabine because it is an oral agent.
Some of my younger patients think of
intravenous therapy as more aggressive,
and they prefer that strategy.
However, this perception is based
on gut reaction rather than reality. I
am a big fan of capecitabine. Maybe it
comes from being a “hormonal therapy
person” who prefers pills to begin with
because I use capecitabine a lot for salvage
chemotherapy in women who have
already had an anthracycline and a taxane
for metastatic disease. In oncology,
we tend to remember our successes, but
I have seen several impressive responses
with capecitabine in dire circumstances.
I have had women on capecitabine for
a considerable period of time with relatively
good quality of life.
Breast Cancer Update 2005 (9)
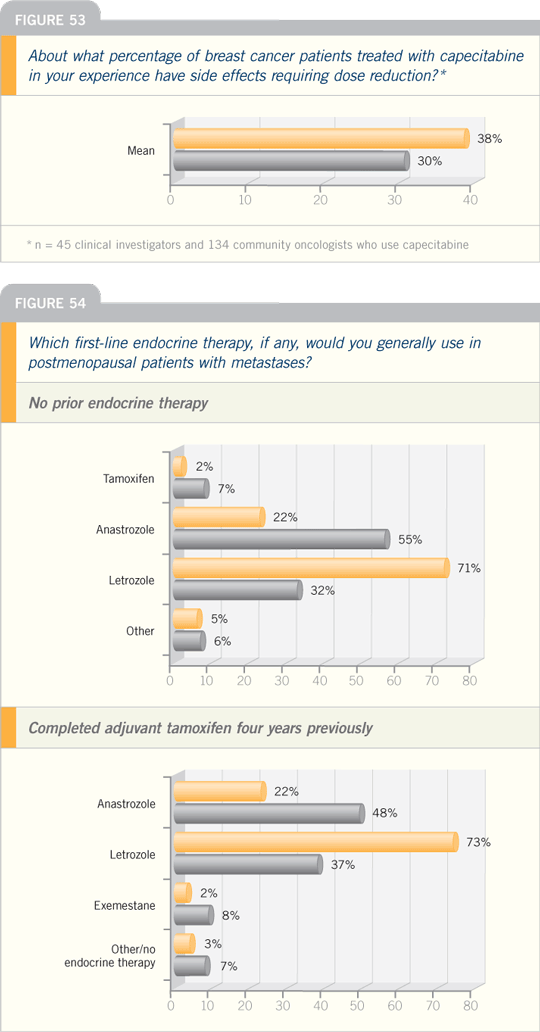
DR VOGEL: In postmenopausal patients,
when I use hormonal therapy in metastatic
disease, for the most part, I generally
start with an aromatase inhibitor.
There are nine lines of hormonal therapy
for postmenopausal women, and there is
no tried and true sequence — we don’t
have any consensus on a true hormonal
cascade. In some women hormones
can be manipulated for years. I’ve had patients on hormonal therapy for 10 or
12 years before ever reaching cytotoxic
chemotherapy.
Breast Cancer Update 2005 (4)
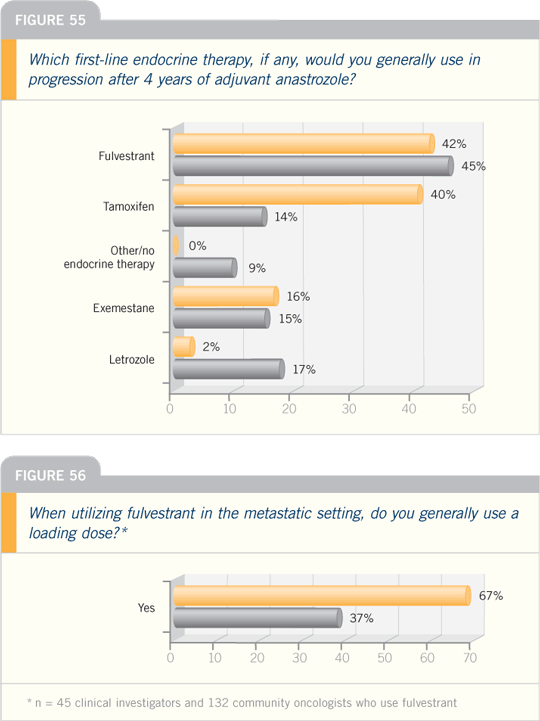
DR GRADISHAR: If you evaluate most of
the available data with endocrine agents
in the metastatic setting — tamoxifen,
steroidal or nonsteroidal aromatase
inhibitors or fulvestrant — the question
that comes up is whether one sequence
enhances patient outcome more than
another. This becomes important
because if you can demonstrate that one
sequence enhances the time to disease
progression, it may be built on over time
so that overall outcome is improved.
In theory, simply having an improvement
in recurrence or progression of
metastatic disease impacts quality of
life. Patients now typically receive a
nonsteroidal aromatase inhibitor —
anastrozole or letrozole — as the first
treatment.
The question then becomes, if patients
progress on one of those agents, what
would be the next best therapy? Should
it be the steroidal aromatase inhibitor
exemestane, or should it be fulvestrant?
Indirect data evaluating the sequence
of a nonsteroidal aromatase inhibitor to
fulvestrant suggest that 25 to 30 percent
of patients may benefit with that
approach. An important issue is whether
fulvestrant at 250 mg is optimal.
Some of the data suggest that the
dose is really on the low end of the
curve where you might expect the optimal
response rate. Some strategies have
evaluated quickly increasing serum levels
of fulvestrant, including administering
loading doses of 500 mg and within
two weeks administering another 250
mg and then proceeding to the monthly
schedule. Those strategies are based on
mathematical modeling that has shown
an ability to achieve steady-state levels
much more quickly and consequently
achieve a biologically relevant dose of
drug circulating much faster.
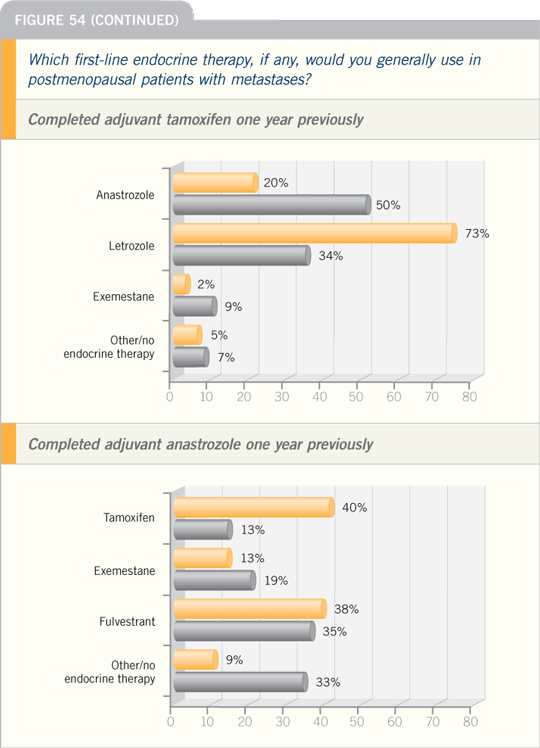
Patterns of Care 2005 (1)
DR JONES: Generally, patients are either
going to relapse on tamoxifen or after adjuvant tamoxifen. In that setting and
in the fulvestrant versus anastrozole clinical
trials, evidence exists that a proportion
of women have a longer response
to fulvestrant than to anastrozole
when given right after tamoxifen. I‘ve
had patients with long responses to
fulvestrant.
I prefer fulvestrant to an aromatase
inhibitor after tamoxifen because
approximately 20 percent of patients
have long responses with it in this setting.
However, 99 percent of oncologists
will choose an aromatase inhibitor after
tamoxifen. Fulvestrant is generally being
used as third-line therapy.
Despite Trials 20 and 21, most physicians
start with anastrozole rather than
fulvestrant because of the way the data
have been presented. We are just beginning
to see patients who have been treated
with two or three years of adjuvant
anastrozole and then relapsed.
Currently, there are few data on treatment
options in this setting. It’s somewhat
of a “dealer’s choice” because there
are no hard and fast rules. There are
multiple options including fulvestrant,
exemestane and even tamoxifen — if the
patient hasn’t seen it — because it’s obviously
still a useful drug. So the sequence
is going to be all over the map for most
folks.
Patterns of Care 2005 (1)
DR BURSTEIN: Previously, patients
received tamoxifen in the adjuvant setting,
so we would use an aromatase inhibitor
as front-line therapy in the metastatic
setting. Fulvestrant was used second line,
or we could use megestrol acetate, but
for many women fulvestrant has a more
convenient side-effect profile.
Now that more women receive
aromatase inhibitors in the adjuvant setting,
we’re using tamoxifen or fulvestrant
as first-line treatment in the metastatic
setting.
Breast Cancer Update 2005 (5)
DR DEBU TRIPATHY: In the up-front
study, tamoxifen and fulvestrant were
essentially equivalent. As second-line
therapy, fulvestrant seemed to perform
equally as well as anastrozole. At this
point in time, the sequencing and timing
for fulvestrant are unclear. I think it’s
reasonable to use the drug — maybe
not up front, but as second- or third-line
therapy.
This is when you might consider
the patient’s preferences in terms of an
intramuscular or an oral drug. A recent
study of 261 women with metastatic
breast cancer demonstrated that about
one third preferred a monthly intramuscular
injection. I’ve always assumed
that oral drugs were preferable, if they
were equally effective. Therefore, I was
surprised to see that many patients preferred
an intramuscular injection. I need
to query my patients more when I start
evaluating these options.
Patterns of Care 2005 (1)
DR JOANNE L BLUM: In my experience,
patients tolerate the fulvestrant injections
just fine. We have randomized data
comparing fulvestrant versus anastrozole
in patients who have already received
tamoxifen, but the optimal sequence for
using fulvestrant is still undetermined. In choosing between an aromatase inhibitor
and fulvestrant, I ask my patients
whether they prefer an injection or a pill.
If they have transportation problems,
then I use an oral agent.
However, for the Medicare population,
these drugs are very expensive. If the
patient does not have adequate insurance
coverage and can’t afford them, a monthly
injection may be better. Compliance
is also an issue to be considered when
choosing between a daily oral agent and
a monthly injection.
Special Edition BCU: Proceedings
from Two Medical Oncology Educational
Forums, 2005
DR OSBORNE: Two clinical trials were
conducted comparing fulvestrant versus
anastrozole for second-line therapy in
patients who had received tamoxifen in
the adjuvant or metastatic setting. One
study was conducted in North America
and the other in Europe and the rest of
the world. The data from both trials were
similar. The complete response rate was
slightly higher with fulvestrant, whereas
the partial and objective response rates
were similar.
In terms of stable disease and clinical
benefit, fulvestrant was a tiny bit better
than anastrozole. In one of the trials,
duration of response favored fulvestrant,
but by and large, the drugs were similar.
How does fulvestrant compare with
tamoxifen in the front-line setting?
All the preclinical data suggested that
fulvestrant would be significantly better
than tamoxifen, so a trial was conducted
comparing these two endocrine
agents. In the receptor-positive group,
fulvestrant and tamoxifen were similar in
response and time to treatment failure,
but overall, tamoxifen looked slightly
better in some of the parameters.
Breast Cancer Update 2005 (8)
DR VALERO: Our approach in the institution
is to use an aromatase inhibitor
up front and then fulvestrant as second-line
therapy. Fulvestrant is approved for
patients who have failed tamoxifen, so
you can use one agent or the other.
In the palliative setting, I believe
you can use it either way. Fulvestrant
has been shown to be as effective as
anastrozole and tamoxifen. I use them
in sequence. I don’t believe there is any
information that one sequence is better
than the other. I use an aromatase
inhibitor, and then I use fulvestrant as a
second-line therapy.
Breast Cancer Update 2005 (8)
DR BUDD: I tend to use an aromatase
inhibitor first and then use fulvestrant.
One could build a rationale for using an
alternative sequence, but I believe the
data for aromatase inhibitors are strong.
When trying to decide between
fulvestrant and an aromatase inhibitor
for a postmenopausal patient who has
relapsed on adjuvant tamoxifen, I believe
ease of administration and the magnitude
of the information are key. We
have trials with each one of these agents
that indicate, in one way or another, that
the aromatase inhibitors are an optimal
treatment. Granted, anastrozole and
fulvestrant appear to be equivalent in
that situation. So fulvestrant is also a
reasonable choice.
I believe most, but not all, patients
would still rather take a pill than have an
intramuscular injection. Many of these
patients are coming back to the clinic on
a monthly basis for a bisphosphonate, so some of the practical advantages of a pill
may not pertain for all patients.
Breast Cancer Update 2006 (3)
DR JOHN FR ROBERTSON: The SoFEA
trial compares exemestane to fulvestrant
following another aromatase inhibitor.
The EFECT study is also testing
exemestane versus fulvestrant following
another aromatase inhibitor. That study
has finished recruiting and is now in the
follow-up phase, so the results should be
reported in the foreseeable future.
With the SoFEA trial, I hope we see
an improvement by combining the two
treatments, though I suspect we may
have answers to that question before the
SoFEA trial results are reported, in that
metastatic studies often take a bit longer
to run. A couple of ongoing studies are
also combining therapies, and they may
report sooner.
The SWOG-S0226 trial is comparing
fulvestrant with anastrozole to
anastrozole alone, so we may see whether
the combination is better than a single-agent
aromatase inhibitor, and that will
be an interesting result.
Breast Cancer Update 2004 (6)
DR MITCHELL DOWSETT: EFECT is
an American and European study that
randomly assigns patients who have failed
therapy with a nonsteroidal aromatase
inhibitor to fulvestrant or exemestane.
Our own study, SoFEA, is slightly
different from EFECT because it is
based on the observation that the addition
of small amounts of estrogen to cells
that have been estrogen deprived for a
long time reduces the effectiveness of
fulvestrant. That scenario equates to the
withdrawal of a nonsteroidal aromatase
inhibitor and the addition of fulvestrant.
Hence, the third arm of our trial
includes a nonsteroidal aromatase
inhibitor and fulvestrant. I predict
fulvestrant alone will probably be better
than exemestane, and fulvestrant
with anastrozole will be better than
fulvestrant alone.
Breast Cancer Update 2005 (9)
DR VOGEL: In terms of efficacy,
fulvestrant seems to be equivalent to
anastrozole. Based on data published
this year in Cancer, there seems to be
no difference in overall survival in the
randomized trials of anastrozole versus
fulvestrant.
Fulvestrant is a good drug and a viable
alternative to aromatase inhibitors in
patients who have disease progression
on tamoxifen. We do have to contend
with the randomized trial of fulvestrant
versus tamoxifen, where we expected a
strongly beneficial effect for fulvestrant
over tamoxifen, which was not forthcoming.
There were some subsets where
fulvestrant appeared to be better, but the
overall results were about the same.
Breast Cancer Update 2005 (8)
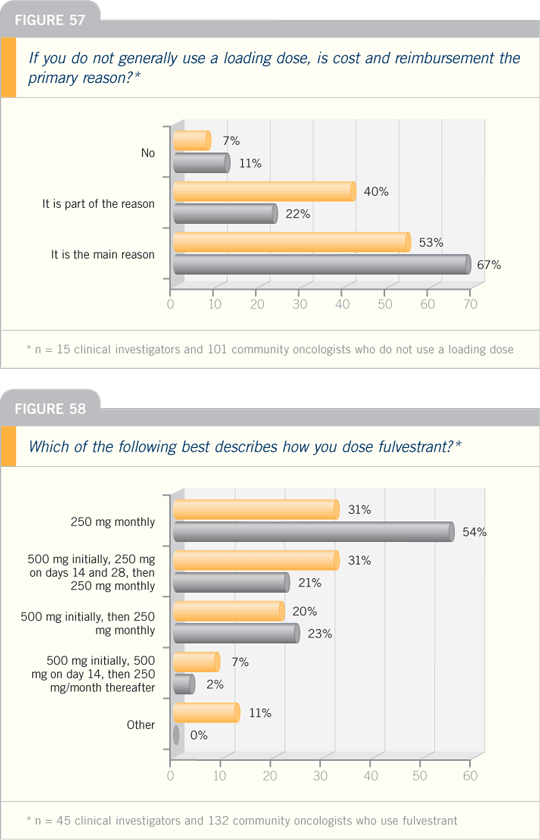
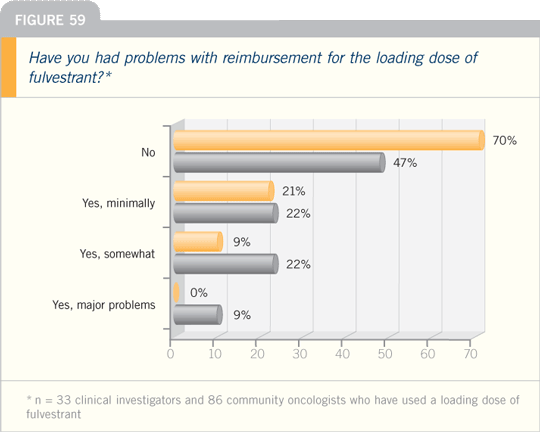
DR VALERO: At MD Anderson we use a
loading dose of fulvestrant. We administer
500 milligrams on day one and 250
milligrams on day 15 and day 29, and
then monthly. Many of the key investigators
in the early development of the drug
believe it is important to attain steady
state. As you know, there are no randomized
data for the loading approach.
Currently, it is FDA approved at 250
milligrams monthly and is reimbursed
by Medicare at that dose. With all of
those caveats, I believe — and I don’t
know if this is my bias — the loading
approach is reasonable.
Meet The Professors 2005 (6)
DR AMAN U BUZDAR: Data suggest
that if you use the package insert dose
of fulvestrant, which is 250 mg every
four weeks, it takes about two to three
months to get a steady-state therapeutic
level. So we give a 500-mg loading dose,
and then in another two weeks we give
another 250 mg, and then treat every
four weeks.
This is being evaluated in a prospective
study because an important question
is whether we are losing some patients
before we get to the therapeutic level
and the disease is progressing because
patients do not have enough drug in their
system.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR ROBERTSON: There is evidence
supporting a loading dose of fulvestrant.
First, tamoxifen reaches a steady state
at two weeks, whereas fulvestrant can
take up to four or five months to reach a
steady state.
Another issue, which I believe makes
people slightly uncomfortable, is that in
the second-line study, fulvestrant was just
as good as anastrozole after tamoxifen.
The first-line study, however, had two
problems. Although it was a randomized
study, 10 percent more people were
assigned to fulvestrant than tamoxifen.
In addition, in the intention-to-treat
population, the time-to-progression
curve for the initial fulvestrant arm drops
down much more quickly than the curve
for tamoxifen, and then, after the first six
months, it runs parallel to tamoxifen. It
makes one think that perhaps the drug is
not on board in that first six months.
The question is, why would you see
this in the first-line and not the second-line
setting? You could argue that some
of those patients in the second-line setting
may be having a tamoxifen withdrawal
effect while the fulvestrant levels
are going up.
Breast Cancer Update 2005 (8)
DR VOGEL: Although we think that may
be utilizing the best dosing schedule for
fulvestrant, we won’t know unless we
do a pharmacokinetic study and a large
study to show that the doses are equally
effective. I’m not sure if we’re going to
be seeing a dosing study large enough to
determine efficacy. You can look at the
pharmacokinetics in a smaller study, but
I don’t know if we’re going to see efficacy
differences.
Fulvestrant is a good drug that has
minimal toxicity. We don’t even encounter
much in the way of buttock pain with
a five-cc injection. We’re also not seeing
the degree of joint discomfort that we see
with the aromatase inhibitors.
Select publications
|
