| |
Adjuvant Endocrine Therapy |
Breast Cancer Update 2005 (8)
DR PETER M RAVDIN: I believe very few
people are being started on tamoxifen
with the intention of receiving five years
of tamoxifen and then switching to an
aromatase inhibitor.
The problem with initially starting on
tamoxifen is that strategies that originally
start with an aromatase inhibitor will
have lower recurrence rates than those
starting with tamoxifen.
If you start a patient on tamoxifen,
you’re conceding that she is going to do
worse initially than she would have done
on an aromatase inhibitor. Then you
have to feel that when you switch her to
an AI, the curves will then recross.
In other words, the aromatase inhibitor
will be so much more effective if
delivered later that it will catch up and
overtake the group that did receive the
aromatase inhibitor from the beginning.
That is possible, theoretically, because
tamoxifen and the aromatase inhibitors
have somewhat different mechanisms of
action.
Therefore, a strategy that uses both
agents might provide the most benefit.
But that’s a theoretical consideration
against the very real fact that we know
if you start with an aromatase inhibitor,
the patients do better.
Breast Cancer Update 2006 (2)
DR KATHLEEN I PRITCHARD: When you
consider randomized studies of up-front
aromatase inhibitors in which disease
recurs more in patients on tamoxifen
than in those on the aromatase inhibitor
in the first two years, it’s difficult
to suggest that you should begin with
tamoxifen.
Until somebody shows in a randomized
fashion that patients who begin
with adjuvant tamoxifen are doing better
at the end of five years than the
patients who use an aromatase inhibitor
initially, I will discuss with virtually all
my postmenopausal patients the idea of
beginning therapy with an aromatase
inhibitor.
Breast Cancer Update 2006 (1)
DR PAUL E GOSS: Two points for upfront
therapy with an aromatase inhibitor
are valid and worth discussing. The
first one is that a slight balloon of events
appears in the first 24 months among patients with receptor-positive breast
cancer. Distant metastases, which are
potentially fatal, occur in the first 24
months at a slightly higher rate than
later.
The second point is that women
treated with tamoxifen face a higher risk
of side effects that are important, particularly
gynecologic intervention, most
of which is inappropriate. Gynecologists
inappropriately intervene in the uterus
for ultrasound-detected thickness that
should be left alone. Nevertheless, the
argument that’s made is that an excess
of events and toxicities makes the choice
of tamoxifen in those initial two years
inappropriate, and you should use an
aromatase inhibitor up front.
The one thing that’s consistent is
that lowering estrogen with letrozole or
anastrozole causes an enhanced bone
resorption. The body tries to prevent
that by compensating with bone formation,
but the net result is bone loss and a
decrease in bone mineral density (BMD).
That is a fact now, but there are a few
caveats. Number one, all of this happened
in an era during which most of the
trials did not specifically recommend the
appropriate osteoporosis guidelines for
calcium and vitamin D supplementation,
which is now being done widely.
Number two, women on aromatase
inhibitors were not adequately screened
with serial BMD tests, which are now
being conducted.
Number three, bone loss very much
depends on whether the woman took
tamoxifen before the aromatase inhibitor
because if she took tamoxifen before, her
bone density is built up above the population
average first and then taken down
by the aromatase inhibitors.
If you look at MA17, for example, at
time zero with five years of tamoxifen,
bone density beats a population age-matched
control. Over the next five
years, it goes below the population age-matched
control, but the net result is
likely to be a return to square one. So it’s
not a massive clinical problem.
I don’t want to understate it; it’s a
real issue, but we have tools to deal with
it. In addition, now that we’re monitoring
more and can introduce the effective
bisphosphonates that we know will
counteract the aromatase inhibitors, I
think we have a tight handle on this.
Breast Cancer Update 2006 (2)
DR ANTHONY HOWELL: All three
aromatase inhibitors are showing about
two to three percent bone loss per year,
and we need to do something about that.
What’s interesting to us is that in the
ATAC data, although the fracture rate
was increased with anastrozole, it leveled
off, and when the patient stopped treatment,
the curves came right back together.
If that’s true, that’s fantastic, but we
need more data to confirm that.
I asked our bone specialists whether
bone density can improve that quickly,
and they pointed out that when steroids
are stopped, bone reforms rapidly; they
were not surprised by our findings.
Breast Cancer Update 2006 (2)
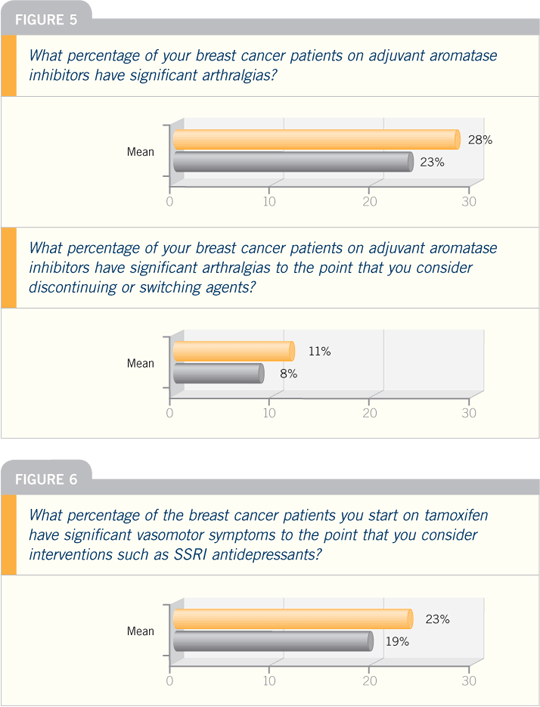
DR PRITCHARD: The absolute difference
between the arthralgias in patients on
aromatase inhibitors versus tamoxifen
is about five percent. I believe the aches
and pains that patients experience with
aromatase inhibitors are real, but it’s
such a peculiar phenomenon.
Some of these women become miserable,
and when you discontinue the
drug, for many, the symptoms disappear.
However, I’ve had some patients that I’ve
put back on tamoxifen, and they still
have the aches and pains.
I think this side effect is related to the
lowering of estrogen. Aches and pains
are reported as a menopausal symptom
and are generally regarded as not all
that common or serious, but maybe we
don’t always listen to what women tell us
about their menopausal symptoms.
With the aromatase inhibitors, we’re
seeing more osteoporosis. In the MA17
data, Goss showed more fractures and
osteoporosis in the patients on the
placebo arm of the original trial who
crossed over to letrozole in the last two
years after unblinding compared to
those who did not.
I think we will see some long-term complications
from this unless these patients
are properly treated for their osteoporosis.
We have to consider how well we
are prepared to either treat our patients
or collaborate with primary caregivers to
prevent osteoporosis, which I think is not
well managed in the general population.
Duffy S et al. The ATAC (‘Arimidex’,
Tamoxifen, Alone or in Combination)
adjuvant breast cancer trial: First results
of the endometrial sub-protocol following
2 years of treatment. Hum Reprod
2006;21(2):545-53.
In summary, this sub-protocol has found
fewer endometrial abnormalities arising
de novo during 2 years of treatment with
anastrozole compared with tamoxifen.
This study also found that endometrial
thickness, as a surrogate marker of endometrial
proliferation, remained consistently
<5 mm in the anastrozole group.
In addition, there was less need for medical
intervention.
Finally, the majority of endometrial
abnormalities occurred in the first year
of treatment. These findings are consistent
with the superior safety profile and
the lower risk of endometrial cancer with
anastrozole compared with tamoxifen as
demonstrated in the main ATAC trial.
Coleman RE et al. Effect of anastrozole
on bone mineral density: 5-year results
from the ‘Arimidex’, Tamoxifen, Alone or
in Combination (ATAC) trial. Proc ASCO
2006;Abstract 511.
Significant bone loss occurred throughout
the five years in the anastrozole
group, although there appeared to be a
slowing down of the rate of bone loss in
years two to five. Although no patients
with normal BMD at baseline had
become osteoporotic at five years, regular
monitoring of BMD and bone protection
strategies are likely to be required
in patients receiving anastrozole in the
presence of pre-existing osteopenia.
Breast Cancer Update 2006 (2)
DR HOWELL: The San Antonio data
from Jakesz are important because they
show that the effect of switching is not
quite as big as we once thought. Whereas
the hazard ratio is approximately a 40
percent reduction in the switching studies,
when they took into account the first
two years, the reduction in the hazard
ratio was about 24 percent.
Another significant finding was the
survival advantage seen in the metaanalysis
of the ARNO 95, ABCSG-
8 and ITA trials. It was an important analysis because it showed, for the
first time in an unselected population,
the survival advantage of switching to
anastrozole after two to three years of
tamoxifen. Based on that, I feel we can
use anastrozole in that clinical setting.
Paul Goss and Jim Ingle’s papers
also presented some beautiful data —
although some of that is selected —
demonstrating the efficacy of letrozole
for patients with hormone receptor-positive
breast cancer.
Combined, I believe these data highlight
the importance of the aromatase
inhibitors therapeutically. We’ve also
seen that apart from the bone events and
aching joints, aromatase inhibitors are
better than tamoxifen as far as toxicity
is concerned.
Robert NJ et al. Updated analysis of NCIC
CTG MA.17 (letrozole vs placebo to letrozole
vs placebo) post unblinding. Proc ASCO
2006;Abstract 550.
With 54 months follow-up the HR for
DFS was 0.31 (0.18, 0.55: p<0.0001)
favoring patients who crossed over to
letrozole compared to those who stayed on
no treatment. The treatment switch was
well tolerated with no significant difference
in bone fractures or cardiovascular
events. ... Women with hormone dependent
breast cancer prescribed letrozole
after a prolonged delay from completing
tamoxifen experienced a significant
improvement in outcome (DFS, DDFS,
OS) and should be considered for this
therapy.
Kaufmann M et al. Survival benefit of
switching to anastrozole after 2 years’
treatment with tamoxifen versus continued
tamoxifen therapy: The ARNO 95 study. Proc
ASCO 2006;Abstract 547.
Median follow-up was 30.1 months.
Switching to anastrozole significantly
improved DFS and OS compared with
continuing on tamoxifen. Fewer patients
who switched to anastrozole reported
serious adverse events (22.7%) compared
with those who remained on tamoxifen
(30.8%). ... Switching endocrine treatment
improved DFS and OS in this well-defined
population.
Postmenopausal women with hormone-sensitive EBC who have already
received 2 years’ adjuvant tamoxifen therapy
should be switched to anastrozole.
Coombes RC et al. First mature analysis
of the Intergroup Exemestane Study. Proc
ASCO 2006;Abstract LBA527.
Switching postmenopausal patients with
receptor-positive or unknown disease
who remain disease free after two to
three years of tamoxifen does appear
to reduce the risk of dying — about a
15 to 17 percent reduction in the risk
of death…serious side effects are rare,
and many may in fact be attributable to
tamoxifen withdrawal.
The switching strategy appears to
minimize the adverse risks of both
agents. Lastly, we conclude that with two
to three years post treatment follow-up,
the only disease related benefits previously
reported appear to be maintained.
We can conclude that exemestane is safe
and well tolerated.
Breast Cancer Update 2006 (2)
DR RAVDIN: Paul Goss presented follow-up
data on patients who participated in
the Canadian trial comparing letrozole
versus a placebo after completing five
years of adjuvant tamoxifen. When they
broke the code at two and a half years,
some of the patients taking placebo
decided to switch to letrozole and a few
chose no further therapy. The patients
who went on to take letrozole had much
lower recurrence rates, although some of
them had been off any endocrine therapy
for four years.
This analysis suggests that even years
after stopping tamoxifen, patients can
gain benefit from an aromatase inhibitor.
In my practice, that means some of
my patients, particularly the patients
at high risk who have already been off
tamoxifen for a year, should consider
taking an aromatase inhibitor, specifically
letrozole, because it’s the only one
that has been tested in this context.
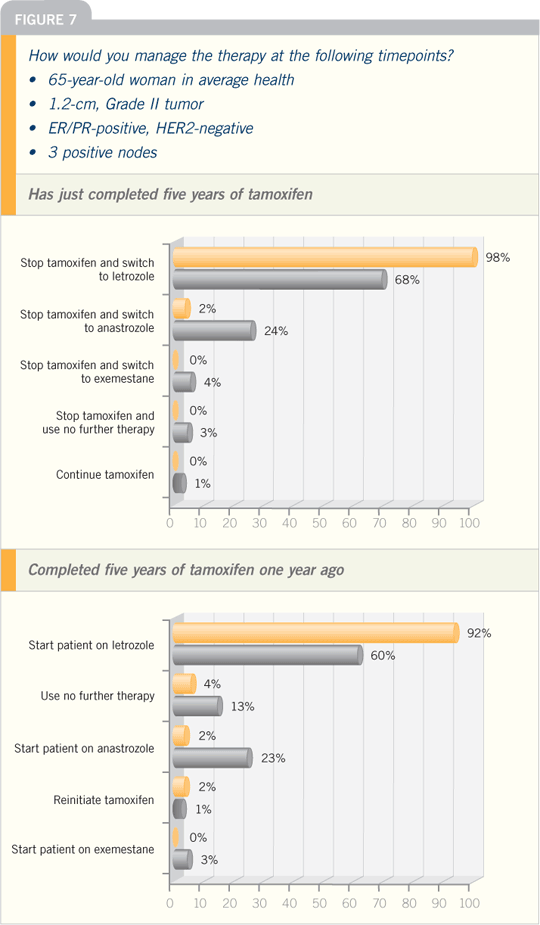
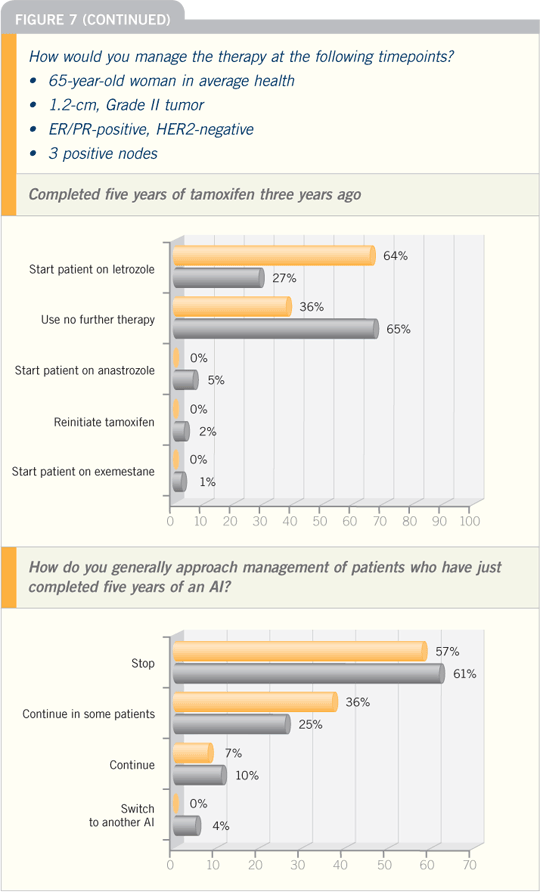
Breast Cancer Update 2006 (1)
DR PRITCHARD: We’re just starting to
see women who have either received five
years of an aromatase inhibitor, or who
switched to an AI after two to three years
of tamoxifen, and we don’t know what to
do in terms of continuing or stopping.
I told the last patient I saw to continue her aromatase inhibitor and come back
in six months because we would have a
clinical trial for her. Both Jim Ingle and
Paul Goss have presented data from the
MA17 trial that suggest, year upon year,
letrozole continues to add benefit.
However, until we see randomized
studies, we’re not going to know the best
way to manage these cases. I think it’s
great that the NSABP is launching a
study to evaluate patients who have had
five years of any aromatase inhibitor or
two or three years of tamoxifen followed
by an aromatase inhibitor.
These patients will or will not then be
randomly assigned to an additional five
years of an aromatase inhibitor.
Breast Cancer Update 2006 (2)
DR RAVDIN: Aromatase inhibition probably
should be continued indefinitely,
and my opinion is based on two
factors. One is that if a patient stops an
aromatase inhibitor, her hormone levels
will, of course, recover.
Second, it may be more difficult to
develop resistance to estrogen deprivation
than it is to develop resistance to
tamoxifen. Tamoxifen is an agonist/antagonist, and preclinical work has
shown that it can be reinterpreted as an
estrogen by cancer cells, but I can’t conceive
of a pathway that would reinterpret
no estrogen as an estrogen.
Breast Cancer Update for Surgeons
2006 (1)
DR J MICHAEL DIXON: In our preoperative
study, we found the aromatase
inhibitors were as effective at reducing
proliferation in patients with HER2-positive disease as in those with HER2-
negative disease. The degree of reduction
was identical in patients with HER2-positive and HER2-negative disease. It’s
as though HER2 isn’t important in relation
to the likelihood of responding to an
aromatase inhibitor.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR C KENT OSBORNE: One important
issue is whether HER2 overexpression
and PR loss predict for less benefit from
tamoxifen than from an aromatase inhibitor.
To me, the data are overwhelming
that PR status predicts for response to
tamoxifen.
In a prospectively designed SWOG
trial published by Peter Ravdin, patients
with metastatic disease were treated with
tamoxifen. The trial was designed to
address the value of PR status. On multivariate
analysis, PR status was found
to be an independent predictor. That
was the first prospective trial following
another five or 10 studies published in
the early 1980s and late 1970s suggesting
that patients with PR-negative disease
responded less well to tamoxifen.
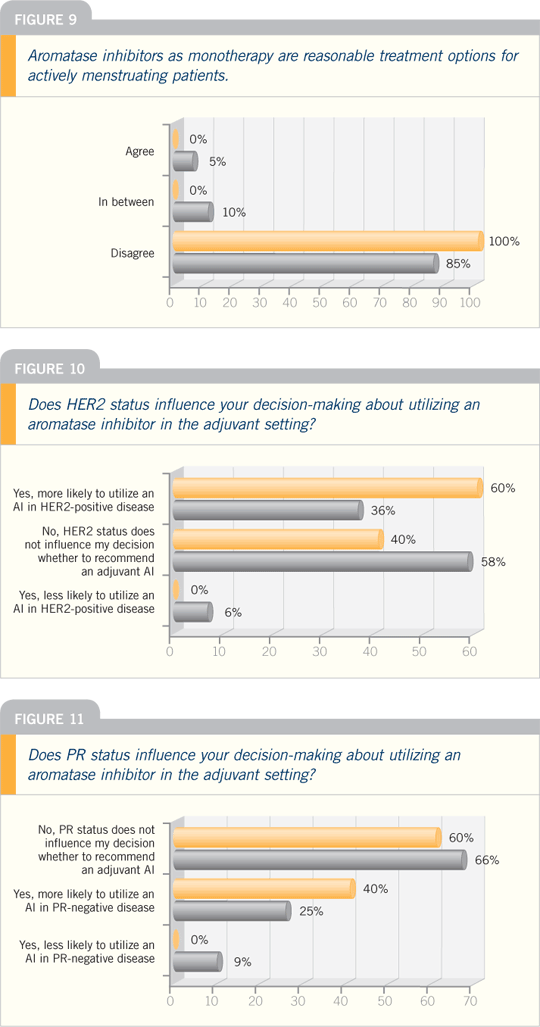
What about HER2 overexpression
and tamoxifen? Most, but not all, studies
show less benefit if HER2 is overexpressed.
Preclinical studies strongly support
the clinical data. So I tend to believe
the majority of the clinical data, along
with the biology, that HER2 does predict
for less responsiveness to tamoxifen.
We have very little data with the
aromatase inhibitors. We have three separate
neoadjuvant trials and a fourth
from Mike Dixon’s group in Edinburgh
that show very similar results. Whether
it is letrozole or anastrozole, the responses
are really quite good for patients with
HER2-positive disease.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR GEORGE W SLEDGE JR: I find the
ER-PR data interesting biologically.
Having said that, I don’t know how
much real-world relevance it has because
I can’t pick out any population of patients
in whom tamoxifen does better than an
aromatase inhibitor.
Because of that, my default — unless it’s going to be the oddball patient who
can’t tolerate an aromatase inhibitor
for some reason — will be to use an
aromatase inhibitor.
Breast Cancer Update 2006 (2)
DR MARTINE J PICCART-GEBHART: I
tend to look at the profile of the tumor.
If I’m dealing with a highly endocrine-responsive
tumor with little worry about
early relapse on therapy — a situation
in which both ER and PR are very high,
the proliferation genes are very low, the
tumor is Grade I, and there is no HER2
overexpression — I believe there is a very
low risk that the patient will relapse if
you put her on tamoxifen for two years.
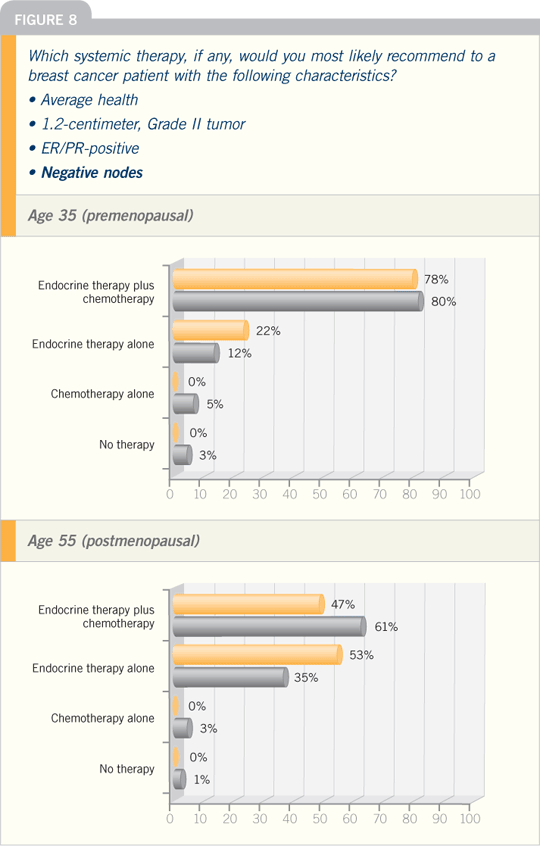
Breast Cancer Update 2006 (2)
DR RAVDIN: The ATAC trial found
that the extra advantage of an aromatase
inhibitor — in this case, anastrozole
— was seen strongly only in the patients
with ER-positive, PR-negative disease.
This finding was based on 6,000 patients,
and it had a very large p-value.
However, the BIG FEMTA study,
which compared letrozole to tamoxifen
as up-front therapy, did not find a significant
difference in the efficacy of these
agents relative to the status of the progesterone
receptor.
The BIG investigators also evaluated
the HER2 status of roughly 4,000
patients because data suggest that
aromatase inhibitors may be more effective
in tumors that are HER2-positive
and ER-positive. They conducted a well-controlled
study and found no significant
difference on the basis of HER2, either.
The relationship between hormone
receptor status and the impact of endocrine
therapy was also examined in the
NCIC-CTG MA17 trial. This trial
randomly assigned patients who had
taken five years of adjuvant tamoxifen
to five years of letrozole versus a placebo.
Patients with ER- and PR-positive disease
particularly benefited from letrozole.
However, patients with ER-positive
but PR-negative disease received no additional
benefit from letrozole compared
to tamoxifen. Interestingly enough, that
observation is exactly opposite to that in the ATAC trial.
At this point, we have no way in
clinical practice to specifically select
patients, and in this state of uncertainty,
an aromatase inhibitor is probably the
better adjuvant endocrine therapy for
postmenopausal patients with ER-positive
breast cancer.
Dowsett M et al. Retrospective analysis
of time to recurrence in the ATAC trial
according to hormone receptor status: An
hypothesis-generating study. J Clin Oncol
2005;23(30):7512-7.
Retrospective subgroup analyses reported
here showed that TTR was longer
for anastrozole- than tamoxifen-treated
patients in both the ER-positive/PgR-positive
and the ER-positive/PgR-negative
subgroups of patients, but the
differential benefit was greater in ER-positive/
PgR-negative tumors. These
data are exploratory, should be considered
hypothesis generating, and should
be confirmed prospectively in other
trials comparing the adjuvant use of an
aromatase inhibitor with tamoxifen.
Breast Cancer Update 2006 (3)
DR KEVIN R FOX: If a premenopausal
woman is treated with chemotherapy
and becomes amenorrheic, it is inappropriate
to assume that she will remain in
a state of real menopause. Based on the
natural history data, it appears to take
two years to establish with some certainty
that a woman will remain in a state of
menopause.
If a 45-year-old woman — five years
from the mean age of menopause —
receives chemotherapy and becomes
amenorrheic, I do not believe we can be
assured that her ovaries will remain in a
state of menopause until we’ve followed
her for two years.
Breast Cancer Update 2006 (3)
DR FOX: The most significant challenge
in developing new adjuvant strategies for
premenopausal women with hormone
receptor-positive breast cancers is the
issue of ovarian suppression. We are
participating in one of the two largest
clinical trials addressing this issue: the
SOFT trial, which randomly assigns
premenopausal women with receptor-positive
cancer to receive tamoxifen
alone, ovarian suppression for five years
with tamoxifen or ovarian suppression
for five years with exemestane.
Breast Cancer Update 2006 (3)
DR FOX: If our patients report two
years of amenorrhea following adjuvant
chemotherapy and we are considering
switching them to an aromatase inhibitor,
we always try to corroborate that information with an estradiol and an
FSH level, recognizing the occasional
shortcomings of either of those measurements.
In my own practice, I require that
a patient have nonmeasurable levels of
estrogen and an elevated FSH level in the
postmenopausal range before prescribing
an aromatase inhibitor.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR ERIC P WINER: In premenopausal
women with ER-positive disease, the issue
of ovarian suppression with an aromatase
inhibitor is being addressed in the SOFT
and TEXT trials. At least some reason
exists to be concerned that this could
possibly be an inferior strategy.
In a woman who has a high level of
estrogen in the premenopausal state, the
estrogen levels go down after she receives
ovarian suppression. Then adding an
aromatase inhibitor and taking a woman
down to extremely low levels of estrogen
may add benefit.
It’s also possible that taking those two
steps down is, in fact, no better than a
single step.
Of course, from a toxicity standpoint
— as I think we’re learning from both
TEXT and SOFT — that deep plunge
into not only menopause but menopause
and an aromatase inhibitor is a
pretty tough maneuver for most of these
patients.
So for premenopausal women, I
would strongly argue against using ovarian
suppression and an aromatase inhibitor
as an up-front strategy outside of a
clinical trial.
What about the use of an aromatase
inhibitor for a woman who is premenopausal
at diagnosis, stops cycling soon
after diagnosis and is now on tamoxifen
for two years? This situation is much
more analogous to the postmenopausal
woman. She has now been without premenopausal
levels of estrogen for two
years. It is more likely that substituting
an aromatase inhibitor for tamoxifen
after two years could be of additional
benefit.
We don’t know that from any of the
clinical trials that have been performed,
but it seems more rational. However,
we’ve all seen in practice — and Hal
Burstein actually has a whole series of
these women — patients who have been
without menstrual cycles for a couple of
years go off tamoxifen and start cycling
again.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR HAROLD J BURSTEIN: The point is
made that amenorrhea is menopause, but
that’s not a very good definition for treating
patients with aromatase inhibitors.
We began to notice some patients — all
of whom were women in their forties
who had chemotherapy-induced amenorrhea
— who were thought biochemically or on strong clinical grounds to be
truly menopausal and were put on an
aromatase inhibitor.
Usually, within six to 18 months they
began to have menstruation again or had
biochemical evidence of residual ovarian
function, suggesting that they were not
obtaining a therapeutic gain from an
aromatase inhibitor.
Smith IE et al. Adjuvant aromatase inhibitors
for early breast cancer after chemotherapy-
induced amenorrhoea: Caution
and suggested guidelines. J Clin Oncol
2006;24(16):2444-7.
Most women older than age 40 treated
with chemotherapy will develop permanent
amenorrhea. However, in a small
minority, reported as 0% to 11%, ovarian
suppression may be temporary, and they may renew menses over time. Our clinical
observations suggest that the incidence
of recovery is probably increased
by the use of AIs (27% in our audit,
compared with 0% to 11% spontaneously
in women older than age 40).
Breast Cancer Update CME 2005
DR ROWAN T CHLEBOWSKI: The data
you have to support using an LHRH
agonist and an aromatase inhibitor for
a younger woman at high risk with a
HER2-positive tumor are a couple of
Phase II trials in metastatic disease with
fewer than 100 patients. So, in a certain
sense, you have very limited information.
Alternatively, you could administer ovarian
suppression and tamoxifen.
That’s what we’ve been doing. You
deviate from these protocols in various
ways. For women under 40 with
hormone receptor-positive disease, we’re
routinely doing ovarian suppression with
tamoxifen. We haven’t utilized the combination
of aromatase inhibitors with
ovarian suppression yet. But I can see
how it may not be an unreasonable
extrapolation to do so.
Breast Cancer Update CME 2005
DR G THOMAS BUDD: Off protocol, in
general, I use tamoxifen for premenopausal
women. Whether ovarian ablation
adds — in terms of efficacy —
to chemotherapy or tamoxifen, I don’t
believe we know. We do know it adds
toxicity.
That’s what ECOG-E3193 in node-negative
breast cancer showed. It’s quite
possible that it will end up adding efficacy,
if we can select the right patient population,
unconfounded by early menopause
from chemotherapy.
Breast Cancer Update CME 2005
DR WILLIAM J GRADISHAR: The data
we have available on ovarian suppression
with tamoxifen or an aromatase inhibitor
are still relatively limited. I would still
view tamoxifen as the optimal therapy.
Select publications
|
