|
Breast Cancer Update 2006 (3):
Miami Breast Cancer Conference Tumor
Panel Discussion
DR SLEDGE: Data from the HERA trial
comparing observation versus one year of
trastuzumab show a significant benefit
in the addition of trastuzumab, with a
risk reduction of about 50 percent and
a strikingly positive p-value. It is interesting
that this trial included no specified
chemotherapy regimen and approximately
one third of the patients had
node-negative disease.
In contrast to the HERA trial, early
analysis of the N9831 trial demonstrated
that the result from the sequential
arm, in which trastuzumab was administered
after completion of chemotherapy,
was not statistically significant, with a
p-value of 0.01. From a purely statistical
standpoint, this did not meet the boundaries
required for early reporting.
The median follow-up in this trial
is short, and the number of events is
small, so which regimen is better is still
an unanswered question. In the arm in
which trastuzumab was administered
concurrently with chemotherapy, the
result was highly significant.
In the BCIRG 006 trial, both of the
trastuzumab-containing arms were superior
to the nontrastuzumab-containing
arm. The nonanthracycline arm may be
minimally inferior to the anthracycline-containing
arm, but this is not yet a
statistically significant difference and
requires further follow-up.
If we examine all these trials as a group and include the FinHER trial, a small
Finnish trial of adjuvant trastuzumab, in
every single study we see significant benefits
with the addition of trastuzumab to
chemotherapy. As a result, trastuzumab
has become the standard of care for
patients receiving adjuvant therapy who
have HER2-positive disease.
Breast Cancer Update:
Special NSABP Edition 2005
DR NORMAN WOLMARK: The only test of
concomitant versus sequential treatment
with trastuzumab was from N9831, and
when you evaluate the curves presented
and the comparisons, one can’t remain
neutral. The concomitant arm (with
paclitaxel) has a hazard rate that falls in
line with what we’re seeing in the other
trials, whereas the sequential arm is,
peer-wise, not statistically significant. It
is not inappropriate for a medical oncologist
to evaluate those data and be more
impressed with concomitant therapy.
Breast Cancer Update:
Special NSABP Edition 2005
DR DENNIS J SLAMON: The initial
BCIRG 006 efficacy data are based on
the first interim analysis of a three-arm
trial with 300 events. We recognize that
we’re walking a fine line here, but still,
both trastuzumab arms crossed their
efficacy boundaries. The relevant question
will be, how does the TCH arm, the
nonanthracycline arm, look relative to
the anthracycline-containing arm?
The risk reduction in the TCH arm
is 0.39, and the risk reduction in the
ACTH arm is 0.51 — almost identical
to what was seen in the trials reported
at ASCO for that type of combination.
That’s based on very few event differences
between the two arms. We need
to wait until the data mature, and it
won’t take a long time. Physicians should
basically do what they feel most comfortable
with at this point. If they feel
more comfortable with the ACTH data,
they should go with that arm, recognizing
that those patients will have to be
watched closely for cardiotoxicity.
In terms of clinical chemotherapy-trastuzumab
combinations, at this
point we try, whenever possible, to
avoid anthracycline-containing regimens
because of the known interaction in
terms of cardiac safety of trastuzumab
with anthracyclines, and we’re not
restricted to TCH when using a nonanthracycline
regimen. There are a number
of different drugs that interact well with
trastuzumab. However, we usually do
use TCH in the adjuvant setting and
will continue to do so until we see that it
is inferior and the safety profile doesn’t
make up for that inferiority.
Breast Cancer Update 2006 (2)
DR BURSTEIN: The seminal question
for the BCIRG 006 adjuvant trial was
how the triplet — docetaxel, carboplatin
and trastuzumab (TCH) — would
compare with AC followed by docetaxel/trastuzumab and whether we could avoid
using an anthracycline.
Although the numbers were not statistically
significant, it struck me that there
is still an advantage for the anthracycline-based
chemotherapy. The trade-off that
Dr Slamon reported is that there seems
to be a slightly greater risk of cardiac
toxicity for the women who received the
anthracycline-based regimen but only
about a one percent difference in terms
of clinical cardiotoxicity events.
I think the findings from the TCH
regimen are provocative, and we should
continue to watch the data as they
mature. However, for the moment I will
continue to use AC followed by a taxane
with trastuzumab as my principal adjuvant
regimen for HER2-positive disease.
Breast Cancer Update 2006 (2)
DR JOHN MACKEY: The intent of the
BCIRG 006 trial was to see if the preclinical
synergy seen between docetaxel,
carboplatin and trastuzumab would be
borne out in the adjuvant setting and
whether we could avoid major problems
with cardiotoxicity by eliminating the
anthracycline.
The trial demonstrated that both the
ACTH arm and the novel arm of TCH
outperformed the control arm, with hazard ratios of 0.49 and 0.61, respectively.
No statistically significant difference
appeared between the two experimental
arms.
Interview, March 2006
DR CLIFFORD HUDIS: We are conducting
a Phase II trial at Memorial with 70
patients treated with dose-dense AC/paclitaxel and trastuzumab. Our goal is
to demonstrate a low or zero incidence of
cardiac events, sufficient to convince us
and the world that this regimen is safe.
We are close to accomplishing that goal;
however, the trial’s not quite finished.
Now that we have evidence that dose-dense
chemotherapy is a little bit better
than conventional chemotherapy,
we don’t want to be in a position of
choosing a less effective chemotherapy
regimen when deciding to administer
trastuzumab. That’s the gap we’re trying
to close.
Breast Cancer Update 2006 (1)
DR PETER A KAUFMAN: We don’t know
the precise approach for the use of adjuvant
trastuzumab in patients with node-negative
disease, but any patient who
meets the protocol-defined eligibility
criteria is a reasonable patient for whom
we should discuss the risks and benefits
of trastuzumab.
I will caution that the number of
patients with node-negative disease in
our NCCTG-N9831 trial was modest — about 10 to 12 percent of the patients
overall. So the findings are way too early
for us to start looking at subsets, but
I think trastuzumab is reasonable for
patients who meet the criteria.
Meet The Professors 2005 (6)
DR WINER: I think the HERA results
are impressive and stand on their own
without a lot of difficulty. It is quite
possible that concurrent may be better
than sequential, but we don’t know at
the moment. The only reason we know
anything from N9831 about sequential
versus concurrent therapy is that when
the DSMV met and decided to release
the data about trastuzumab, as a practice
management question in terms of
what to tell doctors whose patients were
on the trial, they asked to evaluate those
two arms so that they could give doctors
a sense of what to do for patients who
had been treated on the trial and didn’t
receive trastuzumab.
Although there is a statistically significant
difference between the concurrent
and sequential arms on Edith’s trial,
and the sequential arm wasn’t significantly
better than no trastuzumab, it did
not meet any boundary in terms of early
stopping. We just need more data.
We know there’s benefit from HERA.
The risk reduction in HERA was similar
to what we’ve seen in all of the studies.
All of them — other than that one
arm in N9831 — have shown that the
use of trastuzumab either with or following
chemotherapy reduces the risk of
disease recurrence by about half, and the
results are shockingly consistent.
Breast Cancer Update:
Special NSABP Edition 2005
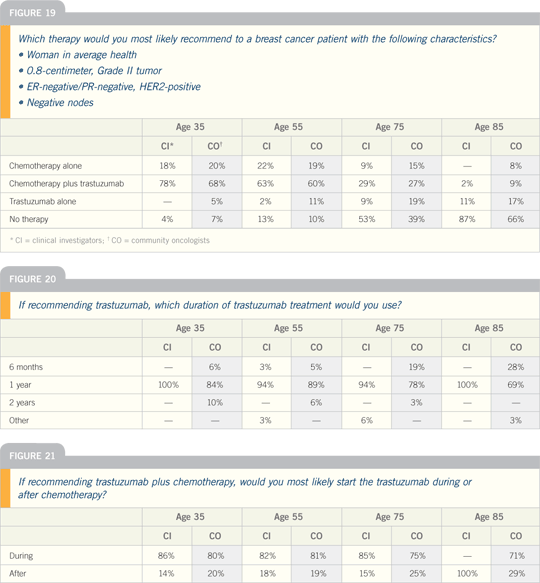
DR WOLMARK: I still have trepidation
about using adjuvant trastuzumab in
patients with node-negative disease and
tumors of less than one centimeter. If
the patient’s tumor is ER-negative, the
threshold to treat with trastuzumab is
lower. However, for those with ER-positive
disease, I would probably want to do
an Oncotype DX™ because I believe that
is a reliable method to determine risk
and would be helpful. If it’s a high-risk tumor, I would add trastuzumab to that
regimen.
Breast Cancer Update 2006 (3):
Miami Breast Cancer Conference Tumor
Panel Discussion
DR JOYCE O’SHAUGHNESSY: If the
tumor is greater than a centimeter in size
and the patient has no contraindications
to trastuzumab, I recommend it. For
patients with tumors less than a centimeter,
it depends on what I think their
residual risk will be. For example, if a
tumor is eight or nine millimeters but ER-negative
and PR-negative, Grade III and
HER2-positive by FISH, I recommend
trastuzumab. For patients at higher risk,
I always use AC followed by paclitaxel
or docetaxel with trastuzumab, but for
patients at lower risk, I consider TCH
because it has less cardiac toxicity.
Breast Cancer Update 2006 (2)
DR PICCART-GEBHART: Women with
HER2-positive tumors were allowed to
enter the HERA trial if the tumor size
was greater than one centimeter. This
was the only criterion. We didn’t require
other aggressive features — it was purely
based on pathological size.
Of course, now the problem we have
is with young women coming to us
with eight-millimeter tumors and negative
nodes. Usually, when you look at
the pathology, you see other features of
aggressiveness — for example, a high
proliferation rate, Grade III tumors and
so on.
I don’t see why these women would
not derive a substantial benefit from
trastuzumab. Provided these women are
well informed about cardiotoxicity risk,
we are discussing with them the possibility
of trastuzumab.
Breast Cancer Update 2006 (2)
DR SANDRA M SWAIN: In NCCTG-N9831,
about 10 percent of the patients
had node-negative disease, and none of
those patients had tumors that were less
than a centimeter. Probably one of the
questions I am asked the most right now
is, “What do you do with a patient who
has a three-millimeter, HER2-positive
tumor?”
In BCIRG 006, about 30 percent
of the patients had node-negative disease,
and some of those patients did
have very small tumors. There was not
a size limitation, but it’s still, again,
limited in number. In the HERA trial,
approximately 30 percent of the patients
had node-negative disease. The HERA
trial was very strongly positive for efficacy
with sequential trastuzumab in the
patients with node-negative disease.
Hence, strong data support the use of
adjuvant trastuzumab in patients with
node-negative disease. The nuances of its
use are really about the tiny tumors.
The data indicate that a patient with
a two- or three-millimeter tumor has an
extremely good prognosis. I have not been
recommending adjuvant trastuzumab
for those very tiny tumors because of
the risk of cardiac toxicity. It’s not like
tamoxifen, with which you have minimal
risk. You do have risk, and it requires
intravenous therapy for a year.
Breast Cancer Update 2006 (1)
DR KAUFMAN: We don’t know whether
women with a HER2-positive tumor
smaller than one centimeter need adjuvant
trastuzumab. We do need to be
respectful of the fact that these women
have a better prognosis because their
tumors are so small. For women
whose tumors are ER-positive and less
than one centimeter, I’ve not offered
trastuzumab.
For patients with ER-negative disease,
I suppose one could consider
trastuzumab, although the quantifiable
gains from adding this agent are not
known. It would be interesting to conduct
a study evaluating trastuzumab with or
without chemotherapy in patients with
very small tumors.
Maybe we can begin to eliminate
chemotherapy for the lower-risk patient
population if we can alter the natural
history of their disease. Data exist suggesting
that trastuzumab may enhance
the apoptotic function of a number of
cytotoxic agents.
Dennis Slamon and Mark Pegram
did much of the groundbreaking work
demonstrating synergistic interactions
between trastuzumab and a number of
conventional cytotoxic agents that we
use widely in breast cancer — docetaxel,
paclitaxel and vinorelbine.
So if that preclinical finding was accurate,
one would speculate or hypothesize
that administering trastuzumab concurrently
with cytotoxics might give you a
much greater bang for your buck and
much more clinical activity than waiting
until the completion of chemotherapy to
start trastuzumab.
In terms of the difference between
sequential therapy versus no therapy
with trastuzumab, we found in N9831
a 13 percent relative improvement in disease-free survival favoring sequential
therapy that did not achieve statistical
significance. Our data indicate a trend
but not a definitive improvement or benefit
with sequential therapy.
The HERA trial, which also looked
at sequential therapy, did demonstrate
a highly statistically significant
improvement with the administration of
trastuzumab in sequence with chemotherapy,
after its completion.
Time will tell. My prediction is that
a benefit does exist with trastuzumab
administered sequentially after the completion
of chemotherapy. The HERA
trial data are impressive, and it’s a large
study.
Click image below to enlarge
Meet The Professors 2006 (1)
DR HUDIS: I have not treated any patients
with adjuvant trastuzumab monotherapy, but if I’d ever be tempted, it would be
for an older patient with a high degree
of ER-positivity. Data for such cases are
desperately needed and would fill an
important gap. Frankly, the remarkably
consistent impact of trastuzumab raises
the question of the contribution of the
chemotherapy.
Breast Cancer Update 2006 (1)
DR SWAIN: I probably wouldn’t use adjuvant
trastuzumab without chemotherapy.
Even if there’s comorbidity, paclitaxel
can be tolerated very well if you use it
weekly.
Such a large amount of synergy data
exists with trastuzumab, even though
the HERA trial is positive with sequential
use, I believe Dennis Slamon’s laboratory
data that indicate the synergy is
important.
So I would try to use paclitaxel with
trastuzumab in those patients, and if
you’re concerned about the anthracycline,
just don’t use that.
Breast Cancer Update 2006 (1)
DR CHARLES E GEYER JR: Right now,
I would shy away from using adjuvant
trastuzumab monotherapy, although it’s
easy to come up with scenarios where one
would do that. The real rigid position is
that we just don’t know what trastuzumab
does by itself or with hormonal therapy.
However, the magnitude of what
we’re seeing with adjuvant trastuzumab,
particularly in the HERA trial on which
it was administered by itself, makes it
difficult to take that position.
If I have a patient who is older and I
don’t want to use TCH or doxorubicin,
seeing the TCH data and knowing that
weekly carboplatin and weekly paclitaxel
are very well tolerated, I would be more
inclined to make that sort of substitution.
If somebody is healthy enough that
I want to provide her with the benefits
of adjuvant trastuzumab, I would tend
to recommend something like weekly
carboplatin/paclitaxel rather than avoid
chemotherapy altogether.
In terms of delayed trastuzumab, the
HERA data suggest that trastuzumab
as monotherapy beyond adjuvant treatment
has a very low risk to it. So from
the patient advocacy perspective, I see
little downside to offering patients a year
of trastuzumab if you know they have a
substantial annual residual risk, which,
for me, would be patients with node-positive
disease.
The more difficult question is, how
far out? I don’t have an answer. It would
be hard for me to justify it beyond five
years. I think at that point patients have
had enough time that they’re likely to be
beating the odds, so to speak.
It looks as though sequential AC followed
by concurrent taxane/trastuzumab
probably presents an increase in significant
cardiac events of about three percent
over baseline.
The cardiac events, as an endpoint in
our study, meant that the patients died
from cardiac causes or developed New
York Heart Association Class III or IV
heart failure. They experienced symptoms
of heart failure with normal activity
or at rest.
We will have to continue to follow the
patients on these large adjuvant studies
longer to obtain information regarding
the long-term effects of trastuzumab
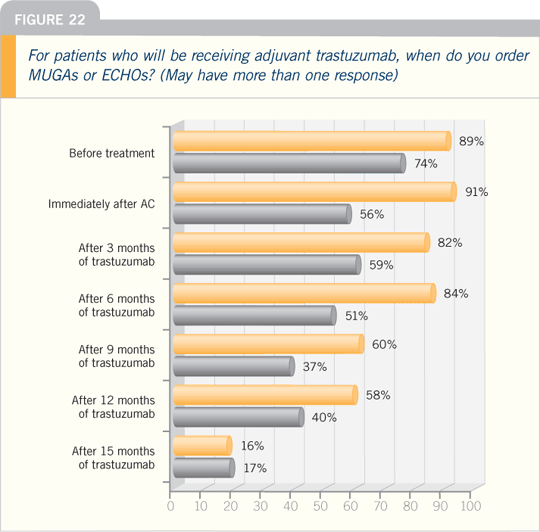
after treatment is finished. NSABP-B-31 and NCCTG-N9831 were designed
to check left ventricular function at 18
months, which was three months after
trastuzumab ended and substantially
longer from when the chemotherapy
ended.
We are seeing encouraging results
there, in that over time the slight decrements
in ejection fraction across the
groups diminish, and we do not see a
great deal of difference looking at median
ejection fractions. Even among the
patients who did develop cardiotoxicity,
in which their ejection fractions dropped
to 30 percent or less, virtually all are up
at least to 40 percent.
Recovery clearly occurs. It will take
time to see what happens four and five
years out. We also noted that relatively
few new cardiac events are happening beyond the end of trastuzumab treatment.
In BCIRG 006, they reported a 1.3
percent event rate with TCH versus 2.3
percent with AC followed by docetaxel/
trastuzumab. The difference was one
percent.
The sequential AC taxane regimens
have a cardiac event rate of about one
percent. When you throw trastuzumab
into that mix, you’re probably adding on
two or three percent.
I don’t think there’s a big difference
between these numbers in terms of the
absolute rate. I don’t think that AC
paclitaxel/trastuzumab is more cardiotoxic
than AC docetaxel/trastuzumab.
We reported four percent; they reported
two percent, but these are different trials.
Cardiotoxicity with TCH is less; they
reported an incidence of 1.3 percent. In
the HERA trial with trastuzumab by
itself following doxorubicin, it was 0.5
percent. It’s interesting to see the TCH
at the level of the ACT — both of them
higher than what HERA was reporting.
It’s always problematic to compare
absolute numbers across protocols, but
the HERA number was strikingly the
lowest number. If you want safety to be
your dominant concern, then the HERA
regimen seems, based on the data, to be
the safest.
Breast Cancer Update 2006 (1)
DR GEYER: When we performed our
cardiac safety analysis, we looked for
predictors of the cardiac events. The
things that held up in multivariate analysis
were the age going in and the post-AC
LVEF. For instance, patients 50 years
old and older who had a post-AC ejection
fraction of 50 to 54 percent had a
cardiac event rate of 20 percent in our
study. The lower boundary of that confidence
interval was 11 percent, so that’s a
very high number.
Based on our data and what I’ve seen,
I think the post-AC ejection fraction
provides an extra measure of safety, coupled
with the fact that it does seem as if
no matter how trastuzumab is administered,
it provides substantial benefits. So
if I can do that and minimize toxicity, to
me, that’s important.
Slamon D et al. Phase III randomized
trial comparing doxorubicin and
cyclophosphamide followed by
docetaxel (ACT) with doxorubicin and
cyclophosphamide followed by docetaxel
and trastuzumab (ACTH) with docetaxel,
carboplatin and trastuzumab (TCH) in HER2
positive early breast cancer patients: BCIRG
006 study. SABCS 2005;Abstract 1.
With over 23 months of follow-up, the
primary endpoint of disease-free survival
in both experimental arms of BCIRG 006
was achieved. The secondary endpoint of
overall survival is not yet mature enough
to report differences.
The cardiac safety data show a statistically
significant higher instance of cardiac
events in the ACTH arm compared
to either the ACT or the TCH arm,
but also importantly, there are persistent
LVF declines in the anthracycline-containing
Herceptin arm compared to
the non-anthracycline Herceptin arm
TCH.
For the global safety, they were tolerated
well. Nonhematologic toxicity was
evenly distributed, and all three regimens
were well tolerated. The final observations
are that the LV declines sustained
with ACT and ACTH do last up to 550
days at the point of the last follow-up for
a significant number of these patients.
Coamplification of the TOPO II gene
with HER2 may identify a subset of
the HER2-amplified that might benefit
from anthracycline, making it worth the
risk of cardiac dysfunction. Conversely,
65 percent of the patients do not have
TOPO II amplification, and they may
be ideal candidates for an efficacious
nonanthracycline-containing regimen.
Smith I, on behalf of the HERA Study
Team. Trastuzumab following adjuvant
chemotherapy in HER2-positive early
breast cancer (HERA trial): Disease-free
and overall survival after 2 year median
follow-up. ASCO 2006.
In conclusion, trastuzumab following
adjuvant chemotherapy significantly
improves overall survival among women
with HER2-positive breast cancer. The
disease-free survival gain reported after
the one-year median follow-up is maintained
after two years’ median follow-up,
and the risk of cardiotoxicity remains low.
Long-term follow-up will provide continuing
safety data, important information
on duration of trastuzumab treatment
— one year versus two years —
and, perhaps, information on the effect
of delayed switching to trastuzumab in
the observation arm.
Select publications
|
