|
Breast Cancer Update 2006 (2)
DR PICCART-GEBHART: I believe that
in the not-too-distant future, we will
approach the choice of chemotherapy
completely differently. We used to think
according to risk, dividing the choice of
chemotherapy regimens into the most
appropriate for patients with node-positive
versus node-negative disease.
We are going to move away from
that because we are entering an era in
cancer medicine with the development
of superb tools to predict which tumors
respond to which drug.
We are not there yet, but this is going
fast. The technologies are exploding. If
we, the clinicians, are smart enough to
design the right studies to validate these
technologies quickly, it’s going to change
the picture.
Instead of our habit of thinking that
six positive nodes means dose-dense chemotherapy,
we should look at the profile
of the tumor first. After that we can
look at the nodes because the number of
nodes is related to risk.
I would never administer dose-dense
chemotherapy to a patient with a Grade
I, highly endocrine-responsive tumor
with maximum receptors and a very low
proliferation index. On the contrary, if I
see a young patient with negative nodes
but an aggressive tumor with absolutely
no endocrine receptors whatsoever, no
HER2 and very high proliferation, I
would be tempted to use dose-dense
therapy.
Martin M et al. Adjuvant docetaxel for
node-positive breast cancer. N Engl J Med
2005;352(22):2302-13.
In BCIRG 001, a randomized, phase 3
trial of adjuvant chemotherapy in women
with operable node-positive breast cancer
showed that, at a median follow-up of 55
months, the estimated rate of disease-free
survival at 5 years was 75 percent
in the TAC group and 68 percent in the
FAC group (P = 0.001). The relative risk
of death was 30 percent lower among
women in the TAC group than among
those in the FAC group.
Moreover, treatment with TAC, as
compared with FAC, was associated
with a 28 percent relative reduction in
the risk of relapse. The reduction in the
risk of relapse did not seem to be driven
by nodal status or by hormone-receptor
or HER2/neu status.
Perez E. TAC — A new standard in adjuvant
therapy for breast cancer? N Engl J Med
2005;352(22):2346-8.
On the basis of the available data, one
can consider TAC to be a standard of
care, as is the dose-dense regimen of
doxorubicin and cyclophosphamide
followed by paclitaxel, for patients with
resected node-positive breast cancer.
However, the exclusion of patients older
than 70 years and the toxic effects associated
with TAC in the BCIRG trial
cannot be minimized.
With this regimen, prophylactic
growth-factor support is necessary to
ameliorate myelosuppression and febrile
neutropenia. A recommendation for the
selection of one regimen over the other
must await completion of the prospective
National Surgical Adjuvant Breast
and Bowel Project trial B-38, for which
the accrual of data is expected to be complete
in the next few years.
Seidman A. Current status of
dose-dense chemotherapy for breast
cancer. Cancer Chemother Pharmacol
2005;56(Suppl 7):78-83.
Dose-dense trials have demonstrated
that filgrastim facilitated bi-weekly
chemotherapy is feasible. Based on
the landmark results of CALGB 9741,
many groups have adopted this strategy
as a new standard of care. However,
appropriate caution should be applied in
extrapolating these data to any/all regimens
outside a clinical trial setting, since
unanticipated toxicities may emerge.
At Memorial Sloan-Kettering Cancer
Center (MSKCC) and elsewhere, feasibility
trials are either planned or under way exploring dose dense regimens containing other agents (eg, docetaxel). It
is intuitive that patients may be willing
to endure the minor inconvenience
of filgrastim administration to shorten duration of treatment and to gain therapeutically.
Meet The Professors 2006 (1)
DR MACKEY: I believe that TAC without
growth factors is more toxic than
dose-dense AC. We have data from
a trial in which Miguel Martin, in Spain,
treated node-negative patients with TAC
or FAC. Early in the trial they thought,
“Gee, for node-negative disease, TAC
is quite tough,” and they mandated
G-CSF.
At that point, they found that the
tolerability increased dramatically. It’s
not a randomized trial, and it’s an intervention
halfway through, but they found
that not only did the febrile neutropenia
rate drop, but the mucositis, fatigue
and diarrhea decreased as well. In addition,
the quality-of-life decrements that
come with chemotherapy were less after
G-CSF was initiated.
I agree that “naked” TAC without
growth factors is probably tougher than
dose-dense therapy with growth factors.
However, I think that difference would
be much less if you used primary prophylaxis
with pegfilgrastim or filgrastim.
I would suggest that if you were going to
use it, use it with growth factor support.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR CHARLES L VOGEL: In our study,
docetaxel was chosen as a representative
regimen that could cause somewhere
around a 20 percent risk of febrile
neutropenia at 100 mg/m2. All three
endpoints — febrile neutropenia, febrile
neutropenia-related hospitalization and
use of anti-infectives — showed dramatic
improvement with the addition of
pegfilgrastim.
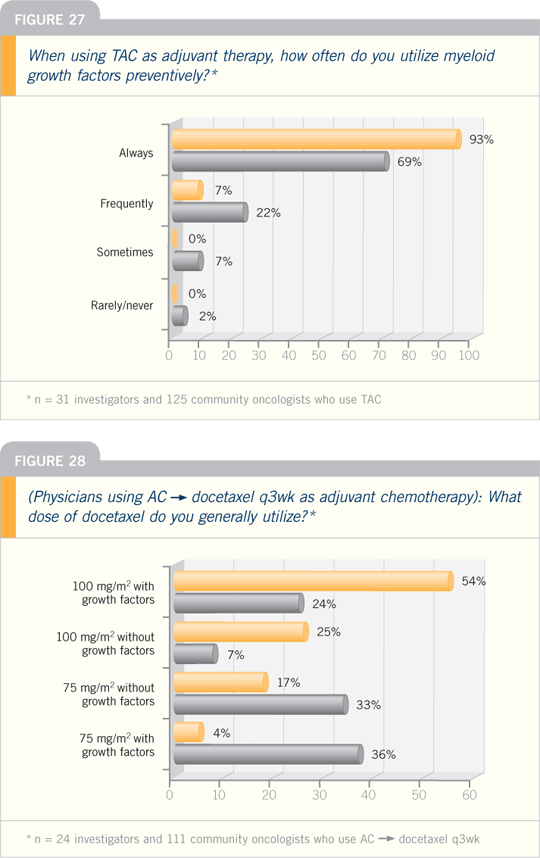
Most people would agree with the
new NCCN guidelines stating that prophylactic
growth factors should be used
for patients with greater than 20 percent
risk of febrile neutropenia. The use of
prophylactic growth factors should also
be considered in the intermediate-risk
group (10 to 20 percent). Patients at low
risk should not receive growth factors.
AC followed by docetaxel, AT and TAC all have very high febrile neutropenia
rates, and prophylactic growth factors
should be strongly considered with these
regimens. AC is considered an intermediate-risk regimen, as is docetaxel/capecitabine.
FAC, FEC and TC are regimens associated
with borderline to low febrile
neutropenia rates. Certainly, dose densification
of any of these would be a
reason to use prophylactic pegfilgrastim,
as would the avoidance of dose reductions
and delays. A third reason to consider
it would be the factors that may
cause patients to be at risk for febrile
neutropenia.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR BURSTEIN: With regard to prophylactic
growth factor support, I believe
most of us would have a hard time
consenting to a regimen associated with
a one in five chance of a patient being
hospitalized with febrile neutropenia
compared to one that wasn’t, simply for
the administration of prophylaxis. So
I don’t find a problem with the recommendation
for prophylactic treatment at
15 to 20 percent risk.
The problem is that we as a community
haven’t defined an acceptable level
of febrile neutropenia. For instance, with
nausea and vomiting, we all agree the
desired goal is zero, so we liberally use
prophylaxis.
For cancer pain, the goal is zero, so we
liberally use pain medicine. We haven’t
said what we’re willing to tolerate in the
way of febrile neutropenia risk.
The only other anecdote I can offer
is that as I administer AC every three
weeks for patients destined to receive
adjuvant trastuzumab, I’m struck by how
many patients end up having dose delays
and tweaks.
It’s clearly more toxic than using dose-dense
AC followed by paclitaxel with
growth factor support.
This hasn’t caused me to use
G-CSF prophylactically in these settings,
but it is impressive how predictable
and clockwork-like every two-week AC
with growth factor support is compared
to other treatments.
I believe if you asked patients whether
they would take a growth factor for a
four percent decrease in their chance of
febrile neutropenia, they’d all say yes.
Whether that is cost effective is a totally
different question.
Breast Cancer Update 2006 (4)
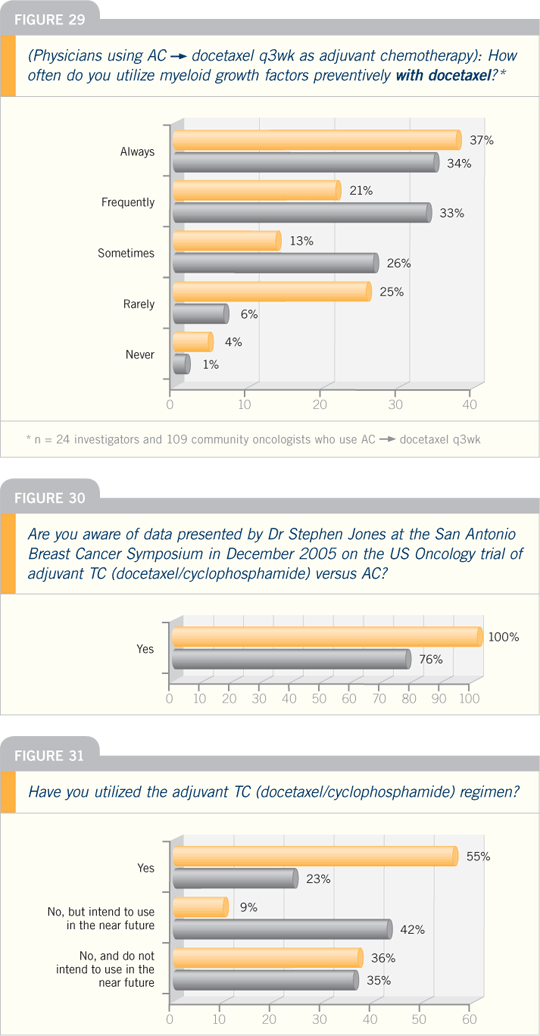
DR STEPHEN E JONES: At the San
Antonio Breast Cancer Symposium in
2005, we reported on a US Oncology
adjuvant study in which we compared
four cycles of standard-dose AC to four
cycles of standard-dose TC (docetaxel
and cyclophosphamide). Chemotherapy
was administered before radiation therapy or tamoxifen, and we included
patients with node-positive and higher-risk
node-negative disease.
When we started this trial in 1997,
everyone was interested in combining
doxorubicin with the taxanes, but we
felt that we didn’t have enough data
to combine docetaxel with doxorubicin.
Consequently, we pursued this alternative
route, which stands alone because it
is one of the only nonanthracycline-containing
regimens out there.
We now have mature results based
on more than 170 events, with a median
follow-up of 5.5 years. We have seen significantly
fewer recurrences and events
on the TC arm compared to the AC arm.
I emphasized at San Antonio that the
endpoint for this trial was disease-free
survival, not overall survival. Overall survival
was the secondary endpoint.
At five years, the disease-free survival
was 86 percent for TC versus 80 percent
for AC — a six percent absolute difference.
The reduction in risk was roughly
one third, and it was highly significant,
with a
p-value of 0.015. Also, a strong
trend was favoring TC for overall survival
— a three percent absolute difference
at five years, with approximately a
24 percent reduction in the odds of dying
from breast cancer.
In general, TC was better tolerated.
Some low-grade docetaxel-type
side effects do occur, such as myalgias,
arthralgias and edema, but they are fairly
transient. The fever and neutropenia
rates are also slightly higher; the numbers
were 5.5 percent on the TC regimen
and 2.5 percent on the AC regimen. We
didn’t use any prophylactic growth factors,
but prophylactic antibiotics were
used and encouraged.
The rate of congestive heart failure
(CHF) with AC was probably lower
than would be expected. The usual figure
that’s quoted is 0.5 to 1.0 percent;
fortunately we haven’t seen that kind of
rate. We have no reason to believe that
TC would cause cardiac toxicity.
AC brought significantly more Grade
III/IV nausea and vomiting, despite
antiemetics. That’s an unpleasant side
effect we didn’t see with TC. I was amazed at how much better tolerated
TC was than AC.
Click image below to enlarge
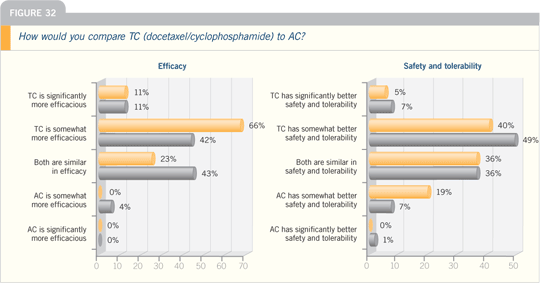
Breast Cancer Update — Think Tank
Issue 1, 2006
DR RAVDIN: In the data comparing
docetaxel/cyclophosphamide to
AC presented by Steve Jones at San
Antonio in 2005, the hazard ratio for
recurrence showed a 24 percent proportional
advantage in survival for docetaxel/cyclophosphamide, which is as big a step
as we usually take in our clinical trials,
and it showed a 36 percent improvement
in disease-free survival.
I believe the improvement in overall
survival is real, and the correct interpretation
isn’t that it doesn’t show a survival
advantage but that it’s underpowered to
show a 24 percent advantage.
Breast Cancer Update 2006 (3):
Miami Breast Cancer Conference Tumor
Panel Discussion
DR O’SHAUGHNESSY: TC is definitely
better tolerated than AC. What did not
come out in the data set — but, if you
took care of the patients on both arms,
you saw it — was less fatigue with the
TC because docetaxel at 75 mg/m2 is
not particularly fatiguing. With AC, you
can get that kind of prolonged queasiness,
and it “drugs you down” for a week
or so in some patients. TC is much less
nauseating and much better tolerated.
It’s really night and day. I have stopped
using AC now in patients for whom I
was using AC. Now I use TC because of
the six percent absolute improvement in
disease-free survival.
Breast Cancer Update 2006 (2)
DR SWAIN: ECOG-E1199, where the
different schedules and different types
of taxanes were compared, really showed
that the weekly versus every three-week
schedule didn’t make any difference, and
the drug, docetaxel or paclitaxel, didn’t
make any difference. So, in clinical practice,
the best plan is to use whatever
you’re comfortable with.
For example, if you like AC followed
by weekly paclitaxel, that is effective,
or AC followed by docetaxel. I personally
would use every three-week instead
of weekly docetaxel. Basically, what
ECOG-E1199 says is that we have a lot
of different options.
Breast Cancer Update 2006 (3)
DR RAVDIN: The ECOG-E1199 trial
asked two questions: Which taxane and
which schedule are optimal as adjuvant
therapy? Patients received AC, and then
the standard arm was paclitaxel every
three weeks for four cycles. Another
arm was a substitution of docetaxel for
paclitaxel, and two arms evaluated these
agents in weekly regimens.
If you look at paclitaxel versus
docetaxel, you see no superiority in a
two-by-two comparison between the two
agents. If you look at every three weeks
versus weekly, you see no difference in
efficacy.
However, the devil is in the details,
and as clinicians we all want to know the
one-by-four comparisons. The results are
consistent with what we’ve seen in metastatic
disease.
The weekly paclitaxel regimen was
the best, with almost a 20 percent better
hazard ratio than the standard arm.
Docetaxel, given every three weeks, also
looked somewhat better.
In both of those cases, however, the
difference was a trend and was not statistically
significant. The weekly paclitaxel
arm looked best in terms of overall survival,
but this is a very early analysis not
dignified by p-values.
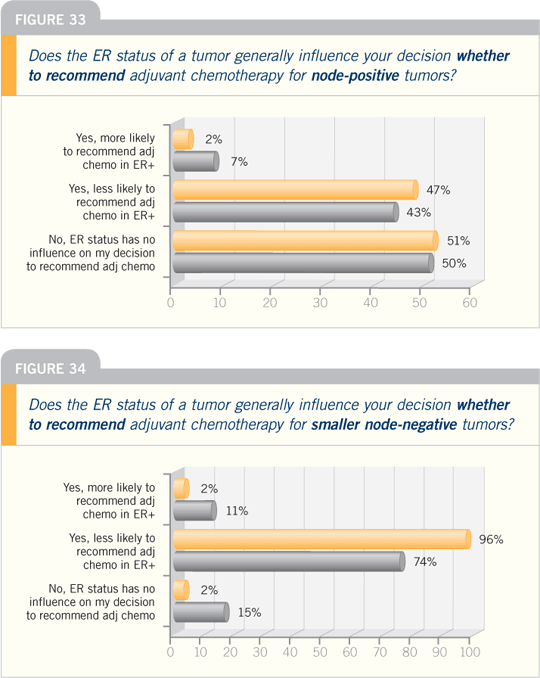
What about toxicity? The weekly
paclitaxel arm seemed to provide additional
benefit without additional risk of febrile neutropenia, whereas the
docetaxel arm was associated with additional
febrile neutropenia.
A conclusion from this study has to
be that weekly paclitaxel in adjuvant
therapy appears promising, and the hazard
ratios for the weekly arm of E1199
looked very similar to those of dose-dense
therapy.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR HUDIS: Don Berry started the
discussion of the impact of ER status on
chemotherapy outcomes in the modern
era by performing an unplanned retrospective
analysis of CALGB trials on the
basis of ER status.
He initially presented his three-study
analysis at San Antonio in 2004 and
compared the high-dose, every four-week
CAF regimen to the standard AC arm of
CALGB-9344. He then studied the AC
paclitaxel arm of 9344 against the standard
arm of the dose-dense 9741 trial.
For patients with ER-negative disease,
the hazard for disease-free survival
was significantly improved with each
one of these steps — better CAF, addition
of paclitaxel, dose-dense scheduling.
Adding up the overall impact for E-negative
breast cancer, we see a profound
chemotherapy effect.
In the subset of patients with ER-positive
disease, the difference in each one of
these steps was not statistically significant,
but they were always favorable.
The point estimate for benefit is half
the size for the patients with ER-positive
disease compared to those with ER-negative
disease. It is likely that it is still
favorable, although the confidence interval
does not exclude the possibility of no
benefit at all.
To some degree, this has been wildly
over interpreted as suggesting that chemotherapy
doesn’t work in patients with
ER-positive disease. It simply doesn’t say
that. It says that the magnitude of the
benefit is likely to be much smaller than
for those with ER-poor disease.
The important point is that when
people say that the addition of dose-dense
scheduling in 9741 doesn’t yield
much among patients with ER-positive
disease, they’re really not comparing
apples to apples when they then look at
the TAC-FAC data.
The TAC-FAC trial demonstrated
hazard rates for risk reductions, which
looked about the same in the ER-positives
and the ER-negatives. The FAC
control arm, of course, includes no
paclitaxel or docetaxel.
You can’t say that each individual step
is or is not significant vis-à-vis another
separate randomized trial. You can’t
compare these regimens head to head.
If you were to argue that you know to
utilize TAC instead of dose-dense AC
paclitaxel in a patient with ER-positive,
node-positive disease, then you’re presuming
to know the results of NSABP-B-38.
I would argue that there is equipoise
on this question and that either regimen
is entirely appropriate for patients with
ER-positive disease.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR WINER: I believe the bottom line is
that if you take all patients with ER-positive
breast cancer, the benefits of
chemotherapy are dramatically less than
in patients with ER-negative disease.
Almost certainly, some groups of
women with ER-positive breast cancer
derive no benefit, and others probably
derive every bit as much benefit as the ER-negative
group. It’s not going to be chemotherapy agent specific, particularly when
we get down to the level of taxanes.
Click image below to enlarge
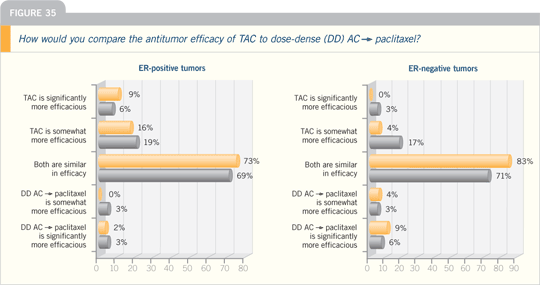

Breast Cancer Update — Think Tank
Issue 1, 2006
DR OSBORNE: The influence of ER
status on the effects of chemotherapy
is such an important question because
60 percent of all patients have ER-positive,
PR-positive disease. Will anyone
conduct a randomized trial of chemotherapy
versus no chemotherapy or endocrine
therapy alone versus the addition of
chemotherapy in that subgroup?
Meet The Professors 2006 (1)
DR HUDIS: Without question, the
Oxford Overview and virtually every
study across the board ever done, including
the TAC/FAC trial, show clear
evidence of a greater magnitude of benefit
for patients with ER-negative disease
who receive chemotherapy than those in
the ER-positive subset.
In the TAC to FAC comparison, the
benefit in the ER-positive subset is statistically
significant. In some of the other
trials, it is not. That trial had the advantage
of centralized ER testing and control
over the hormone therapy.
When we conducted our studies in
the CALGB, we recommended but didn’t
stipulate hormone therapy. We didn’t do
centralized ER testing. So our results are
hypothesis generating. Our hypothesis
is that chemotherapy is, on average, less
effective in ER-positive than in ER-negative
breast cancer. I don’t think that’s a
big stretch.
Whatever small benefit chemotherapy
may offer patients with ER-positive
disease is likely to play out over many,
many years, and you need to have a long
life expectancy to see that difference.
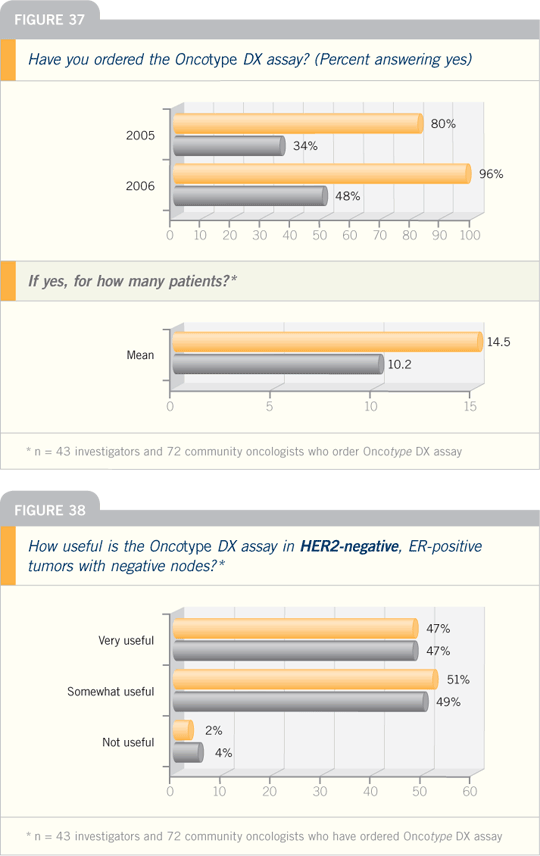
ER-positive breast cancer has a long,
chronic relapse track. Not many people
appreciate this, but by 30 years after
diagnosis, it doesn’t matter whether you
had ER-positive or ER-negative disease,
untreated. The overall risk of relapse and
the overall survival is the same. So the
notion that ER-positive disease is overall
a better disease is undermined.
What’s different is the timing of the
recurrences. With ER-positive disease,
you see the impact of therapy over the
long haul. With ER-negative disease, you
see it right up front.
For a young patient with a long life
expectancy, I wouldn’t be so quick to
forgo that potential benefit. But the
older you get, the longer you have to live
to see the potential benefit and the more
likely you are not to get there.
Breast Cancer Update 2005 (3)
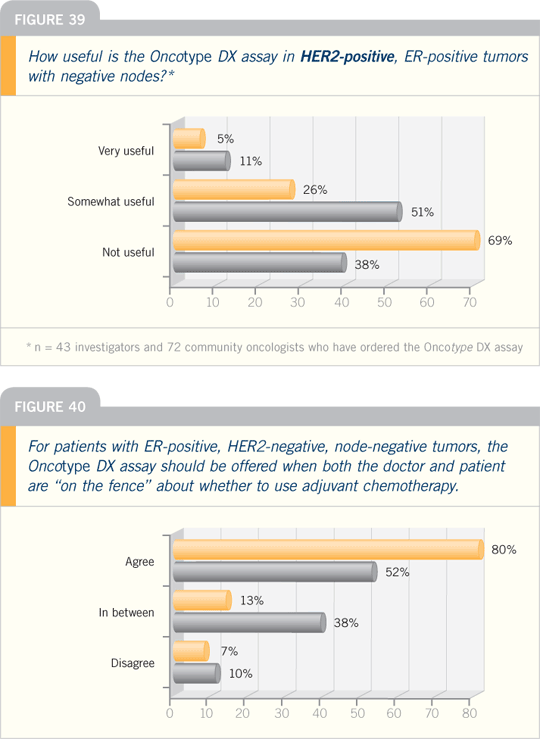
DR SOONMYUNG PAIK: NSABP-B-20
included women with node-negative, ER-positive
disease. It was a three-arm design,
and patients were randomly assigned to
tamoxifen alone or tamoxifen concurrent
with either CMF or methotrexate
followed by 5-FU.
Our study of the Oncotype DX assay
was a retrospective analysis of that completed
trial.
We only had tissue blocks available
for approximately 30 percent of the
entire study cohort, so it’s a subset; however,
the subset and the entire cohort
were comparable.
We repeated the Oncotype DX assay on
the tamoxifen arm to ensure the assay was
reproducible, and we demonstrated that it
is reproducible, which is encouraging.
It is important to note that we evaluated
the NSABP-B-20 chemotherapy
arms to address whether the assay predicted
chemotherapy responsiveness.
We went into that study with an a
priori hypothesis, based on the data
presented at the 2004 ASCO meeting
by Dr Luca Gianni’s group in Milan
evaluating samples from a neoadjuvant
trial they performed with paclitaxel and
doxorubicin.
They demonstrated a correlation
between the Genomic Health recurrence
score and pCR rate.
The higher recurrence rate correlated
strongly with the higher pCR rate.
The overall pCR rate was approximately
25 percent in the patients with high-risk
disease, and there was no pCR in
patients with low-risk disease.
We hypothesized that the benefit
from chemotherapy in NSABP-B-20
would be almost negligible in patients
with low-risk disease and high in patients
with high-risk disease. The results of
this study are striking and unlike anything
I’ve ever seen.
The absolute benefit from chemotherapy
is negative in the low-risk group
and zero in the intermediate-risk group.
In the high-risk group, the absolute improvement in distant recurrence at
10 years is 28 percent, or a relative risk
reduction of 75 percent.
The data in the low-risk group are, in
a sense, not relevant because the baseline
risk after tamoxifen is so low — 6.8 percent
— that it’s a moot point whether
they need chemotherapy.
In the intermediate-risk group the
confidence interval overlaps with one,
so whether patients with intermediate-risk
disease gain any benefit remains a
question.
Breast Cancer Update — Think Tank
Issue 1, 2006
DR DANIEL F HAYES: The patients with
node-negative, ER-positive disease in the
TAILORx, or ECOG-PACCT-1, study
will all be profiled by the Oncotype DX
assay. Those patients with a good recurrence
score of 11 or lower will receive
hormone therapy only.
Those with a high recurrence score of
25 and higher will all receive hormone
therapy and chemotherapy of “dealer’s
choice.”
Those in the intermediate group will
be randomly assigned to receive chemotherapy
or not (investigator’s choice).
They then will all receive hormone therapy,
also at the investigator’s choice.
Meet The Professors SABCS 2004
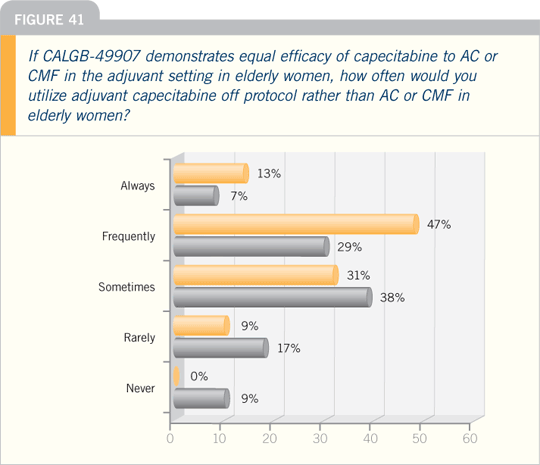
DR HYMAN MUSS: One of the exciting
trials we have ongoing in North
America is CALGB-49907. This is a
trial that essentially compares standard
chemotherapy — four cycles of AC or
CMF with oral cyclophosphamide —
to six cycles of capecitabine for elderly
patients. Physicians can select the standard
chemotherapy for patients randomly
assigned to that arm.
We’re excited about the trial and like
to believe it’s an equivalence study, as
some background data suggest that oral
capecitabine is as good as standard therapy.
It would be nice if we had an oral
regimen because I think people would
rather be at home than in our clinics all
the time.
CALGB-49907 Protocol. calgb.org
A recent randomized phase II trial,
comparing single-agent capecitabine and
CMF as first-line therapy in patients
with metastatic breast cancer who were
55 years and older (median age 69 years),
demonstrated the response rate to
capecitabine alone (25 percent) at a dose
of 2510 mg/m2 per day for 14 days, every
three weeks was superior to intravenous
CMF (16 percent).
Grade 3 or 4 hand-foot syndrome
was seen in 16 percent of patients on
capecitabine and none on CMF, Grade
3 or 4 diarrhea in 8 percent with
capecitabine and 3 percent with CMF,
and Grade 3 or 4 hematological toxicity
in 20 percent with capecitabine and 47
percent with CMF.
In another Phase II randomized trial
comparing capecitabine in the same dose
and schedule as above with paclitaxel 175
mg/m2 every three weeks, the response
rate was 36 percent for 22 patients
on capecitabine and 21 percent for 22
patients on paclitaxel.
These data suggest that the efficacy of
capecitabine in patients with metastatic
disease is similar to CMF or paclitaxel.
Select publications
|
