|

Combined analysis:
NSABP-B-31/NCCTG-N9831
Our conclusions for high-risk HER2-
positive breast cancer: Trastuzumab,
when given concurrently with paclitaxel
following AC chemotherapy, reduces the
risk of a first breast cancer event at three
years by 52 percent. This benefit should
change the standard of care. The benefit
was present and of similar magnitude in
virtually all subsets of patients analyzed.
There is not, however, statistical power
to establish efficacy in the node-negative
subset. The addition of trastuzumab
reduced the probability of developing
distant recurrence by 53 percent at
three years, and the hazard of developing
distant metastases appears, thus far,
to decrease over time. Early results at a
median follow-up of two years show a
statistically significant survival advantage,
with a relative risk reduction of 33 percent.
— Edward H Romond, MD et al.
Presentation. ASCO 2005.
| Breast Cancer Update 2005 (6) |
DR GEORGE W SLEDGE JR: In the
joint analysis of NCCTG-N9831 and
NSABPB-31, the hazard rates for
distant disease recurrence in patients
who received trastuzumab appeared
to improve with time. It’s still early to
analyze these data because few patients
in either trial are four years out; however,
the distant disease-free survival curve
appears to plateau in the trastuzumab
arm. If that’s the case, it’s astonishing.
We’ve never seen a true plateau in
any adjuvant trial. When we examine disease-free survival curves like this, we
need to ignore a fair amount of the right
side of the curve because there are so few
numbers, but if that is maintained it will
be exciting.
HERA: Adjuvant
trastuzumab trial
In conclusion, at one-year median followup,
trastuzumab given every three weeks
for one year following adjuvant chemotherapy
significantly prolongs diseasefree
survival and relapse-free survival and
significantly reduces the risk of distant
metastasis.
Trastuzumab’s clinical benefits are
independent of patients’ baseline characteristics
and of type of adjuvant chemotherapy
received. Trastuzumab therapy
is associated with a low incidence
of severe symptomatic congestive heart
failure, but, clearly, longer follow-up is
needed to better quantify this risk.
All patients continue to be followed
for long-term safety. Results regarding
optimal trastuzumab duration, two
years versus one year, should be available
in 2008.
— Martine J Piccart-Gebhart, MD, PhD et
al.
Presentation. ASCO 2005.
Initial results of BCIRG 006
Breast Cancer Update: Special NSABP Edition 2005 |
DR DENNIS J SLAMON: In a three-arm
trial with 300 events, we recognize that
we’re walking a fine line here, but still,
both trastuzumab arms crossed their
efficacy boundaries. The relevant question
will be: How does the TCH arm,
the nonanthracycline arm, look relative
to the anthracycline-containing arm?
The risk reduction in the TCH arm
is 0.39, and the risk reduction in the
ACTH arm is 0.51 — almost identical
to what was seen in the trials reported
at ASCO for that type of combination.
That’s based on very few event differences
between the two arms. We need to
wait until the data mature, and it won’t
take a long period of time.
Physicians should basically do what
they feel most comfortable with at this
point. If they feel more comfortable with
the ACTH data, they should go with
that arm, recognizing that those patients
will have to be watched very closely for
cardiotoxicity.
Adjuvant trastuzumab in
node-negative disease
Breast Cancer Update: Special NSABP Edition 2005 |
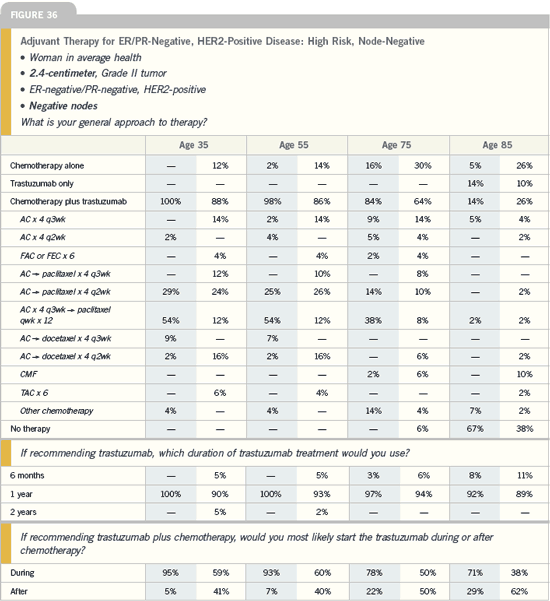
DR WOLMARK: I still have trepidation
about using adjuvant trastuzumab in
patients with node-negative disease
and tumors under one centimeter. If
the patient’s tumor is ER-negative, the
threshold to treat with trastuzumab is
lower. On the other hand, for those with
ER-positive disease, I would probably
want to do an Oncotype DX because I
believe that is a reliable method to determine
risk and would really be helpful.
If it’s a high-risk tumor, I would add
trastuzumab to that regimen.
Breast Cancer Update 2005 (7) |
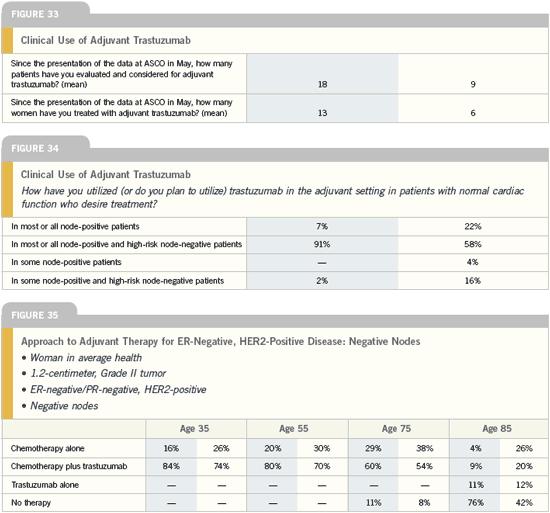
DR ERIC P WINER: The HERA study
included patients with node-negative
disease as long as their tumors were
greater than a centimeter. A third of
the patients participating were node-negative. The NSABP trial had no
patients with node-negative disease, and
in the NCCTG study, patients with
node-negative disease accounted for 14
percent of the total population but only
six percent of the events.
It’s unlikely that the relative benefits
of trastuzumab will differ in patients
with node-negative versus node-positive
disease. On the other hand, the absolute
benefit will differ, because patients
with node-negative disease, particularly
with small tumors, have a lower risk of
recurrence.
In my mind, it’s reasonable to consider
trastuzumab for patients who were eligible
for the studies. The group of women
that I’m a little more cautious about are those with relatively small, ER-positive,
node-negative breast cancers.
Breast Cancer Update 2005 (6) |
DR EDWARD H ROMOND: The NSABPB-
31 and NCCTG-N9831 adjuvant
trastuzumab trials were initially limited
to patients with node-positive disease.
However, in May 2003, the
Intergroup amended their protocol to
include patients with high-risk, nodenegative
disease, which were basically
ER-negative/HER2-positive or ER-positive/
HER2-positive tumors that were
larger than two centimeters.
As a result, in the overall data set,
approximately 100 patients in each arm
had node-negative disease. The relative
risk reduction in the combined data
analysis of patients with node-negative
disease was approximately 0.48 — the
same as the entire data set.
The problem is that with 100 or fewer
patients with node-negative disease in
the arms of the N9831 protocol, the
confidence interval goes out forever and
crosses one.
That does not mean there is no
biologic effect; it probably exists, but
it’s difficult to pin down how much
benefit we gain by using trastuzumab
in patients with node-negative disease.
The HERA trial may be a better data
set to examine the benefit of adjuvant
trastuzumab in that population,
because one third of those patients had
node-negative disease.
Selection of chemotherapy to
combine with trastuzumab
Breast Cancer Update: Special NSABP Edition 2005 (10) |
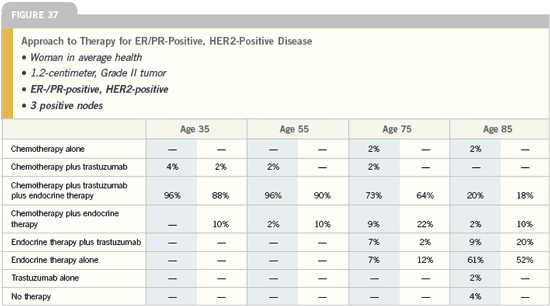
DR SLAMON: In terms of nonprotocol
chemotherapy-trastuzumab combinations,
at this point, we try, whenever
possible, to avoid anthracycline-containing
regimens because of the known
interaction in terms of cardiac safety of
trastuzumab with anthracyclines, and
we’re not restricted to TCH when using
a non-anthracycline regimen.
There are a number of different drugs
that interact very well with trastuzumab.
However, we usually do use TCH in the
adjuvant setting and will continue to do
so until we see that it is inferior and the
safety profile doesn’t make up for that
inferiority.
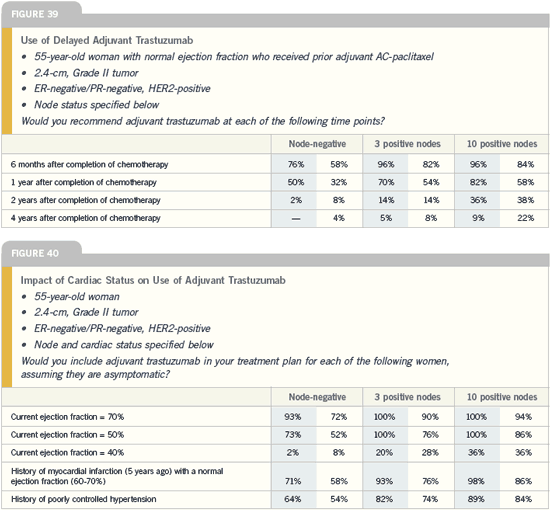
Duration of and delayed
implementation of
adjuvant trastuzumab
Breast Cancer Update 2005 (8) |
DR PETER M RAVDIN: The HERA trial
is evaluating the duration question. In
their trial, one arm has no trastuzumab,
the second arm has one year and the third
arm has two years of trastuzumab after
chemotherapy. Because the data at this
point addresses one year of trastuzumab,
I believe that’s the appropriate length of
time.
As for the delayed implementation
of trastuzumab in the Intergroup trial,
they’re supplying trastuzumab to the
control group of patients who want to
cross over out to one year of follow-up.
There are theoretical arguments that a
year is somewhat of an arbitrary length.
The peak in relapses occurs at about
two to three years, so I could see a rationale
for treating beyond a year, particularly
patients at high risk with multiple
nodes. However, that rationale is going
beyond the data we have and is somewhat
speculative.
Breast Cancer Update 2005 (6) |
DR SLEDGE: The HERA trial suggests
that administering trastuzumab after
chemotherapy may be beneficial, so the
question becomes, how long after chemotherapy
will it be beneficial?
In the case of estrogen receptors, we
have two European randomized trials
that evaluated the late use of tamoxifen
in patients with estrogen receptorpositive
breast cancer, and both were
positive.
Will we see a similar benefit with
delayed adjuvant trastuzumab? It’s a reasonab le
and important question, particularly
for those patients in the control
arms of N9831 and B-31 who are more
than 18 months out from treatment.
I’m not going to be dogmatic about this,
but I do believe it’s reasonable to discuss
the option of trastuzumab with such
patients.
Clinical impact of adjuvant
trastuzumab trial data
Breast Cancer Update 2005 (6) |
DR SLEDGE: As a result of the data
presented at ASCO in 2005, trastuzumab became a standard of care in
the adjuvant setting for HER2-positive
breast cancer.
We saw a stunning validation of the
biology of HER2 and the concept that
we could diminish the likelihood of
recurrence and improve overall survival
through the use of targeted therapy.
This validates 15 years of preclinical
and clinical research, from Slamon’s
initial observation in the late 1980s that
HER2 was a bad actor in breast cancer
to the pivotal metastatic trial led by
Slamon and now the adjuvant trial data.
We have consistently seen that when
HER2 is overexpressed or amplified,
it markedly increases a patient’s risk of
early relapse.
In the HERA trial, we saw that by
two years after randomization, one
quarter of the patients in the control
arm had relapsed. In the joint analysis
of NCCTG-N9831 and NSABPB-
31, around 25 percent had relapsed
by approximately three years. This is a
bad disease, and partly because of that,
we see a high event rate early in these
trials. A striking benefit was seen with
trastuzumab, including survival with a
median follow-up of just two years.
That is unprecedented in any adjuvant
trial. It’s interesting to imagine what
the impact of the estrogen receptor trials
would have been if we had enrolled
3,000 patients on those studies two or
three decades ago. The data probably
would have been similar to the adjuvant
trastuzumab trial data.
The message is that if we understand
biology and target it appropriately,
we obtain a great result, whereas when
we use relatively nonspecific therapies,
we can tweak them — changing dose
duration, dose density and dose intensity
— and obtain slightly better results,
but we’ll never achieve the revolutionary
results that we saw in the adjuvant trastuzumab trials.
Concurrent or sequential
trastuzumab administration
with chemotherapy
Breast Cancer Update:
Special NSABP Edition 2005 (10) |
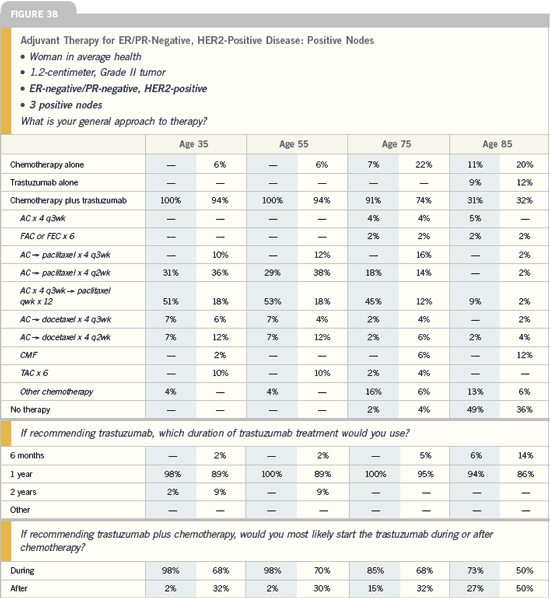
DR WOLMARK: The only test of concomitant
versus sequential treatment was
from N-9831, and when you look at the
curves presented and the comparisons,
one can’t remain neutral. The concomitant
arm (with paclitaxel) has a hazard
rate that falls in line with what we’re
seeing in the other trials, whereas the
sequential arm is, peer-wise, not statistically
significant. It is not inappropriate
for a medical oncologist to evaluate
those data and be more impressed with
concomitant therapy.
Breast Cancer Update 2005 (6) |
DR SLEDGE: In the HERA trial, all
the patients received trastuzumab after
rather than concurrent with chemotherapy,
and those data were positive, with
an impressive 45 percent reduction in
hazard rate. On the other hand, in the
Intergroup trial, it appears that concurrent
therapy is superior to the sequential
schedule. These are different data sets,
and both trials have a short median
follow-up and a relatively small number
of events, so we shouldn’t make too much
of this yet.
Concurrent therapy after the
anthracycline is probably better. I base
that belief on the results of the pivotal trials and on a large body of preclinical
data that suggest trastuzumab is a
good amplifier of chemotherapy-induced
apoptosis. Also, considering how rapidly
the efficacy curves separate in the joint
analysis data, it almost makes one want
to start trastuzumab about 10 seconds
after a core biopsy is obtained.
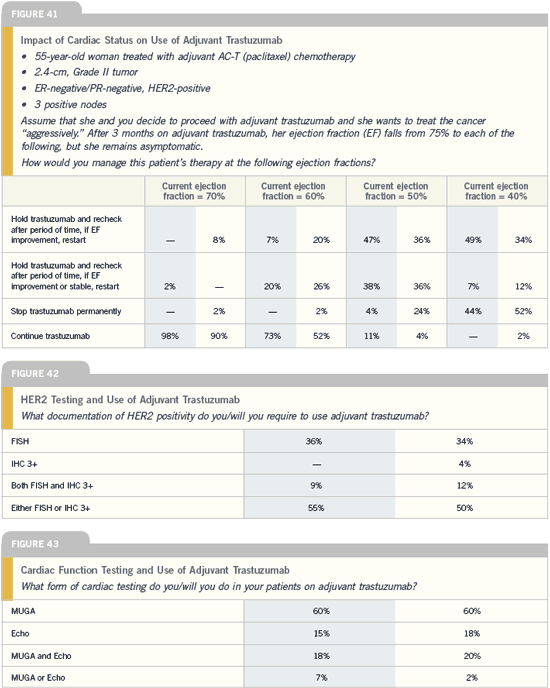
Cardiac safety of
adjuvant trastuzumab
Breast Cancer Update 2005 (4) |
DR EDITH A PEREZ: Although our
trial demonstrated that clinical cardiac
events are observed in patients receiving
adjuvant trastuzumab, the difference is
less than four percent compared to the
control arm. The numbers are actually a
bit lower than the numbers in NSABPB-
31 but statistically quite similar. At
this point, we have not seen any difference
in cardiac events between the two
trastuzumab-containing arms. Not
every patient has a reversal of their cardiac
events, but most patients definitely
improve not only in terms of the clinical
symptomatology but also measurable left
ventricular ejection fraction.
Breast Cancer Update 2005 (7) |
DR WINER: The downside with receiving
trastuzumab, apart from the fact that
it requires a year’s worth of therapy, is
the cardiac toxicity, which was defined
as symptomatic congestive heart failure,
so we’re not talking about asymptomatic
drops in ejection fractions. We’re talking
about real problems that we hope
can improve over time, but about which
we have relatively limited, if any, information
regarding the long-term consequences.
I generally tell patients that the
risk of congestive heart failure is probably
in the range of two to four percent,
based on what we know so far, specifically
in women who receive AC followed
by paclitaxel with trastuzumab.
There was some suggestion that the
cardiac toxicity may be less when trastuzumab
is administered sequentially, as
in the NCCTG trial where paclitaxel
was given and then trastuzumab followed.
Maybe that relates to a longer
period of time from when the
anthracycline is given to the beginning
of trastuzumab. There was also the suggestion
of less cardiac toxicity in the
HERA trial, where chemotherapy and
trastuzumab were not concurrent.
In the NSABP analysis, there was the
suggestion that cardiac toxicity was more
of a problem in older women, specifically
in women who had borderline ejection
fractions at baseline versus those who
had better, stronger, higher ejection fractions.
All of this needs to be sorted out.
In the BCIRG trial, in the group
of women who received docetaxel,
carboplatin and trastuzumab, the cardiac
toxicity was substantially less than
in women who received the anthracycline
followed by docetaxel and trastuzumab.
I think all of us are very hopeful that
nonanthracycline-containing regimens
will be the wave of the future, but we will
just have to wait for the efficacy data. We
need those data from the BCIRG trial,
and I’m told that we will have those some
time over the next several months — certainly
at San Antonio, if not before.
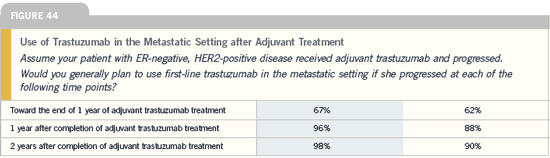
Continuing trastuzumab
after disease progression
Breast Cancer Update 2005 (5) |
DR TRIPATHY: Right now, in the absence
of data, we have to use our best knowledge
and extensions of the clinical and
laboratory data to guide our patients. I
personally believe there may be a role for
continuing trastuzumab with another
chemotherapeutic agent. My patients
also usually feel strongly about it, and we
often elect to go that route. I don’t use
this approach with all of my patients,
and I certainly explain to them that we
don’t know the answer.
In this situation, trastuzumab
appears to be safe. The rate of cardiotoxicity
on the extension trial was very
low in the patients who were already
on trastuzumab and hadn’t developed
cardiac problems. I generally continue
trastuzumab, but not indefinitely. Once
a patient goes through two or three combinations,
I think it’s probably prudent
to stop trastuzumab and try either single-
agent or combination chemotherapy.
Select publications
|

