| |
Editor’s Note: Cool numbers |
Below find some of the more interesting data points that came from this
most recent Patterns of Care survey, completed in August and September
2005 by 45 breast cancer clinical investigators and 100 randomly selected
US-based medical oncologists.
Fraction of breast cancer specialists who
have ordered the Oncotype DX™ assay
(Figure 6, page 10).
This percentage contrasts markedly
with the fraction of community-based
oncologists who have ordered this innovative
assay (34%). The clinical scenario
targeted in related research reported by
the NSABP and Genomic Health is
one of the most common in early breast
cancer — a patient with an ER-positive,
node-negative tumor and, more specifically,
a situation in which both the
patient and physician are unsure about
the value of adding chemotherapy to
endocrine treatment.
Our prior Patterns of Care studies
have documented that this is perhaps
the most common situation in which
clinicians utilize online models, such as
Peter Ravdin’s Adjuvant!. The Oncotype DX assay appears to provide synergistic
information that can be very helpful in
assisting in one of the most vexing decisions
in current medical oncology.
While one might speculate that the
discrepancy in the use of this assay is
based on reimbursement concerns
(Figure 3, page 7), which perhaps are
greater in a community practice setting,
it is also quite possible that researchers
are more familiar with and confident in
the supporting research.
It is particularly plausible that community-
based clinicians may not have
been exposed to the unpublished NSABP
data set reported at the December 2004
San Antonio Breast Cancer Symposium
(SABCS) demonstrating a very dramatic
benefit from adjuvant chemotherapy in
the 25% of patients with high recurrence
scores and no benefit in the remaining
75% of patients with low and intermediate
recurrence scores.
Fraction of breast cancer clinical investigators
who would start a 55-year-old
postmenopausal woman with an ER/
PR-positive, HER2-negative, node-positive
tumor on an aromatase inhibitor
(AI) (Figure 7, page 11).
The optimal long-term adjuvant endocrine
treatment strategy for postmenopausal
women is a topic guaranteed to
generate debate, and at one of our group’s
recent CME meetings, several nationally
recognized investigators revealed that
they start some women with ER/PRpositive
tumors on tamoxifen.
Their rationale is that the ATAC
study showed minimal difference in
relapse rate between anastrozole and
tamoxifen in the unplanned analysis of
this ER/PR-positive subset.
Other AI trials have not reported
a difference based on PR, but a very
common counterargument that invariably
comes up is that even if the antitumor
benefits of these two therapies are
equivalent, AIs show a significant toxicity
advantage over tamoxifen in terms
of thrombotic events and endometrial
cancer.
A number of the “tamoxifen first”
renegade contingency believe that many
breast cancer specialists buy into their
theoretical argument that even though
there are more recurrences during an initial
course of tamoxifen, there might be
fewer recurrences in the long run.
Our survey demonstrates that very
few investigators embrace this approach
in their clinical practices, although when
the case is switched to a woman with
a node-negative tumor, more researchers
(Figure 21, page 18) start with
tamoxifen.
Fraction of investigators who would continue
tamoxifen in a 65-year-old postmenopausal
woman with an ER/PRpositive
tumor with three positive nodes
who has been on tamoxifen for two years
(Figure 10, page 12).
Our previous survey, conducted earlier
this year, demonstrated this same result,
but at that time we noted an important
gap between investigators and community
docs, with 56 percent of community
oncologists continuing tamoxifen.
In our current survey this fraction has
decreased to 24 percent.
Three randomized trials have clearly
demonstrated that patients who switch
to either exemestane (IES study) or
anastrozole (Austrian-German and ITA
studies) have about a 40 percent lower
risk of relapse and experience fewer serious
adverse effects than those who continue
tamoxifen.
Clearly, there is a rapidly building
consensus that five years of adjuvant
tamoxifen is an inferior therapy for postmenopausal
women compared to a treatment
plan that includes or consists of
an AI.
Four years after the first ATAC presentation,
with a number of other AI trials
also reporting advantages for AIs over
tamoxifen, it is clear that an important
change in practice has occurred, and the
minority of oncologists who still use five
years of tamoxifen in postmenopausal
women should re-evaluate their positions
in fairness to their patients.
If I were a postmenopausal woman
with a breast cancer who relapsed after
having received tamoxifen for initial upfront
therapy without having had the
option of switching discussed, I would
be very unhappy. Yes, AIs cost more,
but if an inferior therapy is being recommended to save money, the patient needs
to know that is happening and literally
“buy in” to the plan.
Fraction of patients treated by community-
based oncologists with AIs who
have arthralgias significant enough to
consider discontinuation or switching to
another therapy (Figure 9, page 12).
A key issue in management of these
musculoskeletal symptoms is diagnosis.
A recent report from the ATAC trialists
noted that about one third of patients
on tamoxifen had arthralgias, and while
that number is higher with a significant
p-value for anastrozole, the absolute increase is only about 5 percent.
These data remind us that arthralgias
in a patient on either tamoxifen or an
AI may be caused by something other
than the endocrine therapy. The ATAC
trialists urge that oncologists attempt to
make a precise diagnosis in this situation
and carefully rule out other important
causes of this nonspecific syndrome.
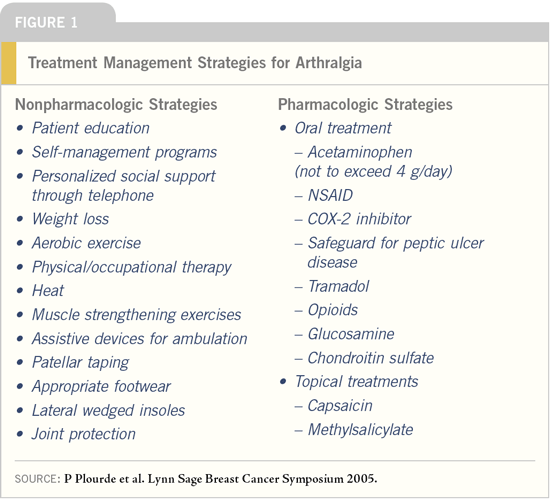
The trialists also urge that physicians
utilize an integrated management plan
(Figure 1) in an attempt to relieve musculoskeletal
complaints before therapy
is switched to another agent (tamoxifen)
that also has the potential for side effects
and toxicities and a greater risk for cancer
relapse. Note that intolerable vasomotor
symptoms are commonly reported
with tamoxifen (25% of patients; see
Figure 9, page 12).
Fraction of breast cancer specialists
who generally utilize dose-dense
AC  T in 35-year-old patients with ERpositive,
node-positive tumors (Figure
23, page 19). T in 35-year-old patients with ERpositive,
node-positive tumors (Figure
23, page 19).
Our prior surveys also demonstrate
that the dose-dense regimen is the one
most commonly used in node-positive
tumors. However, over the past year,
data have begun to emerge from a variety
of sources suggesting less benefit
from chemotherapy in general, and perhaps
dose-dense therapy in particular, in
patients with ER-positive tumors. Our
survey suggests that to this point, docs
have not changed their practices in that
regard.
One change that is emerging in
the selection of chemotherapy is a
switch in the Number 2 spot from
AC docetaxel to TAC. While many
clinical investigators have proclaimed for
several years that TAC and dose-dense
AC T are the two adjuvant regimens
with the best supporting evidence bases,
docs in practice were commonly using
AC T are the two adjuvant regimens
with the best supporting evidence bases,
docs in practice were commonly using
AC docetaxel rather than TAC. docetaxel rather than TAC.
One might guess that the shift this
year away from AC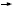 docetaxel to TAC
is attributable to the disappointing
results with that regimen in NSABP-B-
27, reported in December 2004 at San
Antonio. docetaxel to TAC
is attributable to the disappointing
results with that regimen in NSABP-B-
27, reported in December 2004 at San
Antonio.
Fraction of clinical researchers who
would recommend trastuzumab as
adjuvant therapy in younger women
with node-positive tumors (Figure 38,
page 28).
Adjuvant trastuzumab has now clearly
arrived on the scene. Our CME group
has been following emerging clinical cancer
research for more than 20 years and
has never witnessed such a rapid and
complete uptake of a new treatment
strategy.
To put this in perspective: With other
recent advances in the adjuvant setting
— such as the aromatase inhibitors after
the ATAC presentation in December
2001 and the first report a year later of
the effects of dose-dense chemotherapy
in CALGB-9741 — only about a third to
a half of oncologists changed their practices
in the year following the initial key
reports for both of these treatment strategies,
and it took about two years in total
to hit a “steady state” of utilization.
The immediate acceptance and
implementation of adjuvant trastuzumab
reflects several factors, including
the following:
1. Virtually all breast cancer researchers
expected the adjuvant trastuzumab
trials to show benefit.
2. The magnitude of benefit was
impressive.
3. Extremely rapid and efficient methods
of disseminating clinical research
data and perspectives now exist in the
medical oncologist community. (See the
next cool number.)

Fraction of community docs who cited
the Breast Cancer Update audio series
as a source of information about data
from the adjuvant trastuzumab trials
presented at ASCO on May 16, 2005
(Figure 2, above).
In August, when this Patterns of
Care survey was executed, our CME
group had distributed one Breast Cancer
Update audio program discussing the
adjuvant trastuzumab data presented
at ASCO 2005. It is interesting to
note that at the time of the survey, this
piece — which included interviews with
George Sledge and Edward Romond —
had already been heard by almost half of
the oncologists in this country, and perhaps
another 20 to 30 percent are likely
to hear this program and others covering
trastuzumab in the next few months.
Like all contemporary physicians,
medical oncologists learn about emerging
clinical research findings in a variety
of ways. In addition to live events such
as scientific meetings like ASCO and a
mélange of tumor boards, grand rounds
and CME meetings, docs also keep up to
date by the individual use of information
delivery vehicles.
I started my medical education career
as a video producer, and while this medium
was fun and interesting, it requires
the user to focus solely on the program,
which is a problem because docs are busy
as hell and have scarce time to allocate
for continuing education.
Our initial attraction to audio for
oncology CME in the late 1980s was
based on the hypothesis that the ability
to multitask (mainly drive a car or exercise)
while listening would present an
important advantage. Today, we know
from more than a dozen external studies
of our work over the last five years that
about three quarters of oncologists in
the United States are regular listeners to
our programs and that most of this use
occurs in the car.
It’s both awesome and scary to think
that our CME group has the opportunity
to pass on critical clinical research information
such as the adjuvant trastuzumab
data, and we take this responsibility very
seriously.
Fraction of clinical investigators who
start adjuvant trastuzumab concurrently
with the taxane portion of chemotherapy
(Figure 38, page 28).
This finding has a potentially interesting
international twist, and one wonders
if investigators in Europe — where
the HERA trial was executed — have
the same practice pattern as we do in the
United States.
Note that HERA demonstrated
that the post-chemotherapy use of
trastuzumab resulted in an impressive
reduction in risk of recurrence of
about 50 percent, as in the NSABPNCCTG
combined analysis of concurrent
trastuzumab (with taxanes).
On the other hand, the NCCTG
data presented at ASCO revealed a nonstatistically
significant 13 percent reduction
in relapse rate following sequential
therapy à la HERA, while the concurrent
arm of that trial (with paclitaxel)
demonstrated a statistically significant
reduction in relapse rate of 36 percent.
Many oncologists in practice find
these results contradictory, and a third
of these docs currently favor a HERAlike
strategy. The sequential approach
may also lessen the risk of cardiac toxicity,
but that is speculation at this point.
Perhaps the clinical investigators in our
survey are more uniform in their preference
for concurrent therapy because they
feel more confident in their own data.
Fraction of clinical investigators and
community oncologists who would offer
a year of trastuzumab to a 55-year-old
woman treated two years previously for
HER2-positive disease with 10 positive
axillary nodes (Figure 39, page 30).
Most oncologists are considering
delayed trastuzumab, probably because
the clinical research groups who performed
the adjuvant trastuzumab studies
offered that therapy to participating
patients who were randomly assigned to
chemotherapy alone and were less than a
year out from the completion of therapy.
I am very concerned about these
“missed the window of opportunity”
patients, who remind me of the tens of
thousands of postmenopausal women who relapsed or had thrombotic events
while on tamoxifen in the year or two
after the ATAC trial was first presented.
When patients and their loved ones
face the tragedy of distant recurrence,
it is important for them to believe that
they did all they could to prevent it, and
patients who don’t at least hear about
new treatment options like aromatase
inhibitors and trastuzumab — initially
and at delayed time points — are not
receiving state-of-the-art advice.
Click here to see image
Fraction of community-based oncologists
who would recommend bevacizumab to a
40-year-old taxane-naïve patient as part
of first-line therapy in the metastatic setting
(Figure 45, page 34).
The fraction of clinical investigators
who would use bevacizumab and, specifically,
paclitaxel-bevacizumab is almost
double this fraction. At CME meetings
and other venues, many communitybased
oncologists have noted that they
are frankly confused about what to do
concerning the data presented at ASCO
by Kathy Miller on ECOG-E2100, demonstrating
a progression-free and overall
survival benefit when bevacizumab was
added to paclitaxel.
This is particularly interesting in view
of the common utilization of “bev” by
community-based oncologists for metastatic
colon cancer. Part of the issue with
breast cancer is concern about reimbursement
for this non-FDA-approved,
pricey therapy (Figure 3, above), but
perhaps equally important is the skepticism
often found in community docs
when only one trial demonstrates
benefit, particularly in a noncurative
situation.
There also is a general belief among
oncologists that so many options exist
for metastatic breast cancer that the
use of bev seems less compelling than,
for example, in non-small cell lung cancer,
where FDA approval of bev/carbo/
paclitaxel is likely to be followed by a
stampede of use similar to what we saw
in colon cancer.
Fraction of investigators who recommend
capecitabine as first-line therapy for metastatic
disease in a 40-year-old patient
who is minimally symptomatic and had
received prior adjuvant therapy with an
anthracycline and a taxane (Figure 47,
page 36).
As in our prior surveys, capecitabine
is far more commonly utilized by investigators
than community docs. What
is particularly interesting is that half
of the researchers who would utilize
capecitabine would add bevacizumab in
this situation.
A study in the late-line metastatic
setting of this combination — also by
Kathy Miller — did not meet its primary
endpoint of progression-free survival,
although there was an increased response
rate in patients treated with bev. Some
investigators consider this a positive trial,
and others do not. The capecitabine/
bevacizumab regimen was well tolerated,
and cited by Eric Winer in his ASCO
discussion of Kathy’s paper as one of several
considerations for patients who are
not good candidates for taxanes.
Fraction of oncologists who consider the
short infusion time and lack of need for
premedication clinically significant benefits
for the use of nanoparticle paclitaxel
(Figure 53, page 39).
Clinicians are clearly putting their feet
in the water gingerly with this new and
somewhat costly therapy, but one might
expect that as reimbursement issues are
resolved and additional research is generated,
many more patients will be treated
with this interesting agent.
Note that docs are currently split
in terms of weekly versus three-weekly
use of nab paclitaxel (Figure 52, page
39), with the weekly contingent likely basing their preference on Phase II US
Oncology data from Joanne Blum.
Fraction of clinical investigators (and
community docs) who believe that the
efficacy of nab paclitaxel is equal to
or greater than both paclitaxel and
docetaxel (Figure 54, page 40).
Complementing this somewhat uniform
perspective about antitumor efficacy
is the perception that nab paclitaxel
is also better tolerated than the other
two available taxanes. If these oncologists
are correct, it seems possible that
nab paclitaxel will emerge over the next
couple of years as perhaps the dominant
taxane utilized in breast and perhaps
other cancers.
Fraction of investigators and community
docs who would use fulvestrant in
a postmenopausal patient who relapses
on adjuvant anastrozole (Figure 61,
page 46).
This choice is interesting, particularly
because there are no data comparing
tamoxifen to fulvestrant in this situation,
although there are data showing
essentially equivalent results of these
two agents in hormone-naïve patients.
Other research has demonstrated at least
equivalence between fulvestrant and
anastrozole in patients who progress on
tamoxifen.
Note also that about half of the investigators
use a loading dose of fulvestrant,
while this approach is much less commonly
utilized in the community setting
(Figure 64, page 48).
This is our fifth issue of Patterns of
Care devoted exclusively to the management
of breast cancer. With each new
survey, I eagerly anticipate the opportunity
to look at the data because in
my mind these findings truly provide
a glimpse into what clinical investigators
and oncologists in practice consider
state-of-the-art patient care.
I can’t wait to see what cool numbers
emerge in 2006.
— Neil Love, MD
NLove@ResearchToPractice.net
| The data in this monograph reflect a
series of telephone/fax/email surveys
conducted in August/September 2005
of 100 randomly selected US-based
medical oncologists who spend more
than 50 percent of their time in patient
care. Sample sizes of 50 are presented.
In addition the survey was completed
by 45 clinical investigators who spend
more than 80 percent of clinical time
caring for breast cancer patients. Survey
results were reviewed by Clifford Hudis,
MD and Debu Tripathy, MD. |
Select publications
|

