| |
Chemotherapy for
metastatic disease |

| Breast Cancer Update 2005 (1) |
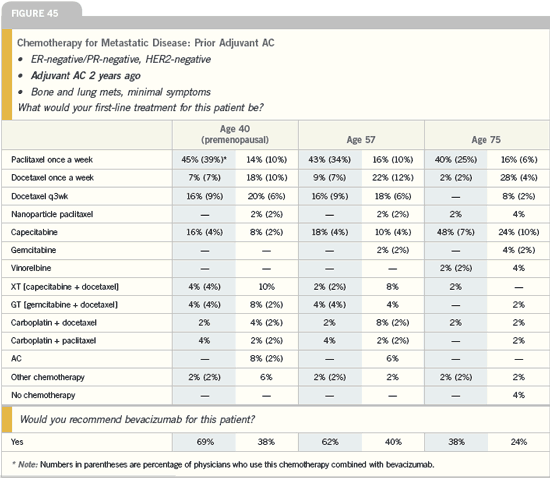
DR JOANNE L BLUM: I decide whether
a patient should receive combination
chemotherapy or sequential single agents
based on the burden and pace of the
disease. For example, women with quite
a bit of visceral involvement — particularly
liver involvement — may need
combination therapy. For the patient
with much more indolent disease, particularly
the patient with a long disease-free
interval who may have had sequential
hormonal therapy and is now hormone
therapy refractory, I use sequential single
agents.
Many of my patients receive capecitabine
as the first chemotherapy in this
situation because it’s orally administered,
does not cause alopecia and is extremely
well tolerated. It is similar to taking a
hormone pill.
| Breast Cancer Update 2005 (5) |
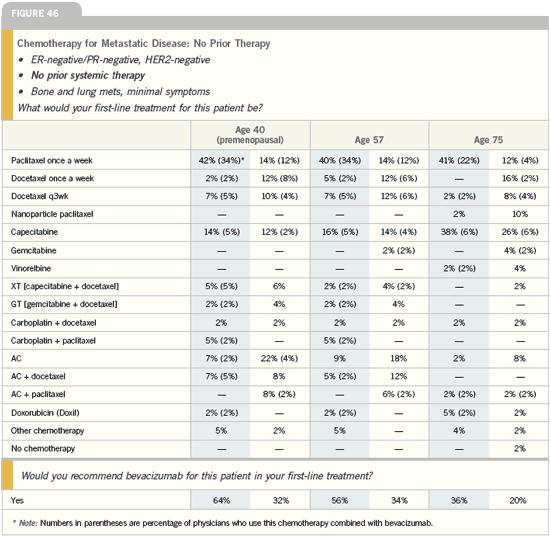
DR NANCY E DAVIDSON: Many times
in metastatic disease, we use all of the
available therapies, so what we’re really
deciding on is the order — what to start
with. Many patients make that decision
based on their personal values.
I find many of my older patients are
attracted to capecitabine because it is an
oral agent. Some of my younger patients
think of intravenous therapy as more
aggressive, and they prefer that strategy.
However, this perception is based on
gut reaction rather than reality. I am a
big fan of capecitabine. Maybe it comes
from being a “hormonal therapy person”
who prefers pills to begin with because I use capecitabine a lot for salvage chemotherapy
in women who’ve already had an
anthracycline and a taxane for metastatic
disease.
In oncology, we tend to remember our
successes, but I have seen several impressive
responses with capecitabine in dire
circumstances. I have had women on
capecitabine for a considerable period of
time with relatively good quality of life.
| Breast Cancer Update 2005 (2) |
DR DICKLER: Positive data exist
for several combinations, including
capecitabine/docetaxel and paclitaxel/
gemcitabine. The doxorubicin/docetaxel
combination improved response rate
but didn’t improve overall survival,
and George Sledge demonstrated that
sequential therapy was as good as combination
treatment in terms of overall
survival, so I tend to use sequential
single agents for the vast majority of
my patients.
In a patient who is chemotherapy
naïve and needs a rapid response, I
would consider an anthracycline-based
combination regimen. It would probably
be doxorubicin/docetaxel, but it
could also be doxorubicin/paclitaxel. If
a patient had dose-dense AC/paclitaxel
in the adjuvant setting, I’d be very
interested in incorporating a gemcitabine-based combination or a capecitabine-
based combination.
I use a lot of capecitabine. I believe it’s
a great drug and is generally well tolerated
when given at nonpackage-insert
doses. For the patient who’s had adjuvant
AC 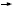 T, I frequently use capecitabine
or vinorelbine as first-line therapy. For
someone who’s chemotherapy-naïve, my
first choice would probably be weekly
paclitaxel followed by either vinorelbine
or capecitabine. I don’t use early-line
doxorubicin up front very often in my
asymptomatic patients, because I think
it causes a lot of fatigue and alopecia.
Weekly paclitaxel also results in alopecia,
but I prefer to use weekly paclitaxel
more than doxorubicin in the metastatic
setting. T, I frequently use capecitabine
or vinorelbine as first-line therapy. For
someone who’s chemotherapy-naïve, my
first choice would probably be weekly
paclitaxel followed by either vinorelbine
or capecitabine. I don’t use early-line
doxorubicin up front very often in my
asymptomatic patients, because I think
it causes a lot of fatigue and alopecia.
Weekly paclitaxel also results in alopecia,
but I prefer to use weekly paclitaxel
more than doxorubicin in the metastatic
setting.
| Breast Cancer Update 2005 (5) |
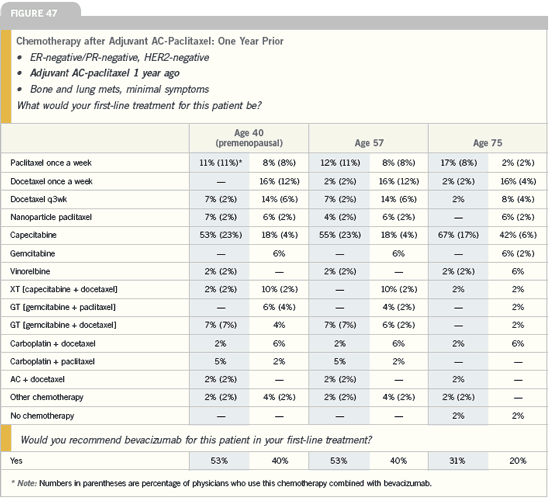
DR TRIPATHY: When we have many
chemotherapy drugs that, as single
agents, provide response rates that
overlap with each other, it shouldn’t be
looked at as a conundrum but rather as
an opportunity to individualize therapy
based on the side-effect profiles. I’m
starting to use drugs with less toxicity
first, because we generally see the longest
duration of response with the drug we
use first. We might as well have that long
period of time be the one with the least
toxicity. Utilizing an agent that does not
result in hair loss should be considered,
if that’s important to the patient. Or, in
the patient with pre-existing neuropathy
from diabetes or prior chemotherapy,
avoidance of an agent with neurotoxicity
is important.
For me, the single most important
factor is what treatment the patient has previously received. If a patient has
progressed on an adjuvant taxane, I’m
more likely to use a nontaxane drug.
Although, granted, you can see responses
to docetaxel and nanoparticle albuminbound
(nab ) paclitaxel upon progression
with the original paclitaxel formulation.
I usually consider patients progressing
after AC and a taxane as being
anthracycline and taxane refractory, but
if a long period has passed (ie, two
or more years) since the adjuvant therapy,
one could certainly retry a taxane.
Nanoparticle paclitaxel or a weekly
regimen of the original paclitaxel formulation
would be attractive choices.
However, I’m generally treating these
patients as anthracycline and taxane
refractory, and I’m using capecitabine.
Not only is capecitabine FDA approved
for that indication, it seems to have
among the higher response rates in
the anthracycline- and taxane-refractory
group of patients. Alternatives to
capecitabine would include vinorelbine
and gemcitabine. I believe combinations
of these drugs are also something to
consider.
We’re so geared toward thinking
of single agents, but combinations
do have a role, particularly for more
symptomatic patients. It’s hard to know
which combination wins out. Data exist on combinations of vinorelbine/
capecitabine, gemcitabine/vinorelbine
and gemcitabine/capecitabine.
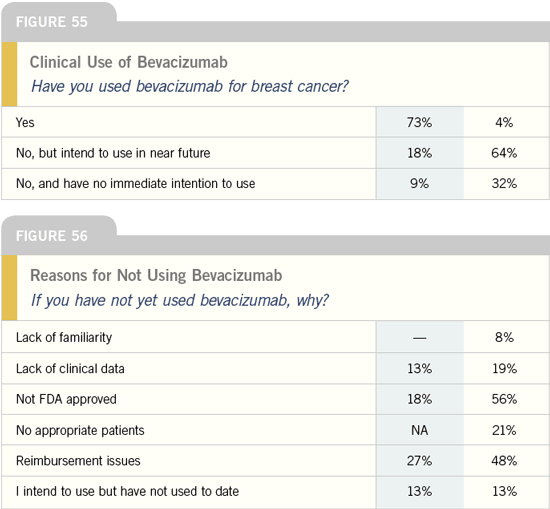
Clinical use of bevacizumab:
Implications of the
results from E2100
| Breast Cancer Update 2005 (6) |
DR SLEDGE: Bevacizumab ought to be
considered for use along with taxanebased
therapy as front-line therapy for
patients with metastatic breast cancer.
I certainly would not argue with those
who suggest we need safety data for bevacizumab
in combination with docetaxel
or nab paclitaxel. However, much of our
preclinical testing was with docetaxel,
and I would expect docetaxel to work
well with bevacizumab.
I would not be surprised if nanoparticle
taxane therapy would also work well. In
fact, the nanoparticle taxanes have — as
a possible mechanism of action — an
effect on endothelial cells. We might
see some synergistic activity there also.
I’m not aware of any safety data for nab paclitaxel in combination with bevacizumab,
but I suspect it would be safe. I
would not have a problem with someone
using the combination, and I would not
expect any unusual toxicity.
| Breast Cancer Update 2005 (7) |
DR WINER: I believe the results of
ECOG-E2100 are impressive enough
that, in the absence of a contraindication
to bevacizumab, I would use it in a
first-line setting, optimally in combination
with paclitaxel as administered in
the study. I doubt that the interaction
is specific between paclitaxel and bevacizumab,
although I’m well aware that
when given with capecitabine in more
advanced disease, bevacizumab seemed
to be less active. However, I believe that’s
probably related to the setting rather
than the drug.
Nanoparticle paclitaxel
compared to other taxanes
The superior efficacy, favorable safety
profile, and greater patient convenience of
ABI-007 [nanoparticle paclitaxel] make
this novel albumin-bound paclitaxel an
important advance in the treatment of
patients with MBC [metastatic breast
cancer]. ABI-007 warrants further investigation,
using additional dosing regimens
(eg, weekly) and in combination
with other treatment modalities, as
front-line treatment of breast cancer and
other solid tumors.
— William J Gradishar, MD et al.
J Clin Oncol 2005;23(31);7794-803.
| Breast Cancer Update 2005 (1) |
DR BLUM: I believe nanoparticle
paclitaxel is more active than paclitaxel
based on the randomized trials. In
cross-study comparisons of nanoparticle
paclitaxel versus docetaxel, each given
every three weeks, the response rates
were similar — in the 30 percent range.
However, docetaxel in the metastatic
setting, whether given weekly or every
three weeks, is toxic because of side
effects like asthenia, fluid retention and
neutropenia, and it’s difficult to administer
for long periods of time.
One can give docetaxel in the adjuvant
setting where treatment is short term,
but I believe nanoparticle paclitaxel is
better tolerated. I don’t use single-agent
docetaxel in the metastatic setting, and
I would use nanoparticle paclitaxel in
lieu of weekly paclitaxel. I would like
to see more data on combinations with
nanoparticle paclitaxel to learn more
about the toxicity profiles before using it
in a combination off protocol.
| Breast Cancer Update 2005 (5) |
DR TRIPATHY: A weekly regimen of
the original paclitaxel formulation would
have been my choice in the past. Now
that we have data with nab paclitaxel, I think that’s a reasonab le option also.
From the data, nab paclitaxel may be
preferable. It outperformed the original
paclitaxel formulation when administered
every three weeks.
A weekly regimen also seems to outperform
an every three-week regimen
of the original paclitaxel formulation,
and I’m left wondering which is the best
drug to use. For patients who prefer an
every three-week schedule, I believe nab paclitaxel is the way to go. Otherwise, it’s
a toss-up between every three-week nab paclitaxel and a weekly regimen of the
original paclitaxel formulation. I don’t
believe there’s a way to compare the
two. CALGB is planning to conduct
a head-to-head trial comparing weekly
regimens of nab paclitaxel and the original
paclitaxel formulation.
Phase III trial of docetaxel
versus paclitaxel
This is the first clinical trial to compare
directly the taxanes docetaxel and
paclitaxel as monotherapy for patients
with advanced breast cancer. Using
US Food and Drug Administrationapproved
doses and schedules for each
agent, this Phase III study has demonstrated
that docetaxel is superior to
paclitaxel in TTP (5.7 vs 3.6 months; p <.0001), response duration (7.5 vs 4.6
months; p = .01), and OS (15.4 vs 12.7
months; p = .03). The overall response
rate was also greater with docetaxel (32%
vs 25%; p = .10). The survival advantage
for docetaxel was observed despite the
increased incidence of toxicities leading
to dose reductions and treatment withdrawal,
and the slightly greater use of
salvage treatment in patients randomly
assigned to paclitaxel. The results of this
study are consistent with those reported
for previous Phase III studies of singleagent
docetaxel and paclitaxel...
— Stephen E Jones, MD et al.
J Clin Oncol 2005;23(24):5542-51.
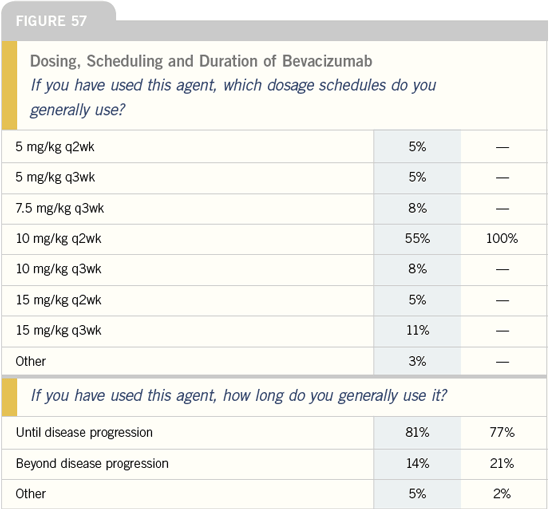
Dosing of bevacizumab
| Breast Cancer Update 2005 (6) |
DR SLEDGE: The dose we choose is based
on our Phase I/II dosing in patients with
breast cancer. Colon and lung cancer
had different paths based on randomized
Phase II trials conducted in those
diseases. I don’t know what represents
the right dose of bevacizumab.
We know 20 mg/kg is too much
because of the dose-limiting toxicity of
migraines. In the Phase I trials, once we
got past approximately one mg/kg, all
circulating, free VEGF was bound. So
somewhere in between one and 20 mg/
kg is the right dose. We used 10 mg/kg
in E2100. In the Phase I/II breast cancer
trial, we looked at doses of three mg/kg,
10 mg/kg and 20 mg/kg. Even though
the numbers were small, we had a sense
that when we went from three to 10 mg/
kg, the responses were more brisk, and
we saw relatively more patient benefit.
Select publications
|

