| |
Adjuvant Systemic Therapy |

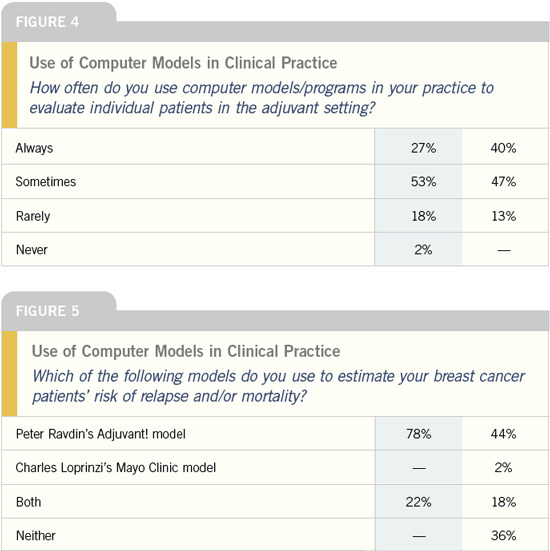
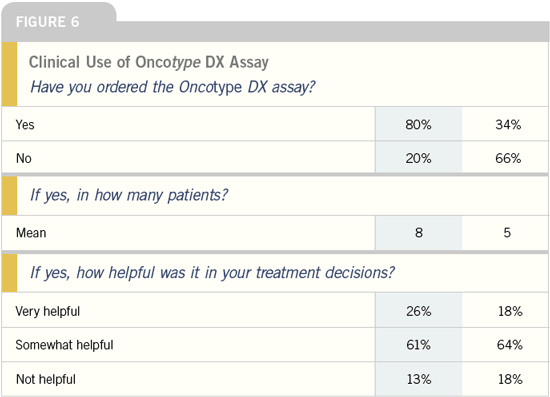
Oncotype DX™ assay
| Breast Cancer Update for Surgeons 2005 (1) |
DR NORMAN WOLMARK: We wanted
to determine whether the Oncotype DX assay could predict the benefit of
chemotherapy, so we examined the data
from NSABP-B-20, which randomly
assigned patients with receptor-positive,
node-negative disease to tamoxifen
versus tamoxifen plus CMF chemotherapy
versus tamoxifen plus MF chemotherapy.
We found that patients who
were at high risk based on their recurrence
scores derived benefit from chemotherapy,
but patients at low risk, who
comprised 50 percent of the cohort, did
not appear to derive substantial benefit
from the addition of chemotherapy
to tamoxifen. The intermediate group
comprised only 20 to 25 percent of the
cohort, and we didn’t have the power to
determine if they benefit from the addition
of chemotherapy.
We were surprised to find that the
relative risk reduction was not uniform
— different risk groups did not have the
same relative risk reduction. The greatest
relative risk reduction was seen in
patients at highest risk.
Computerized models and
the Oncotype DX assay
| Breast Cancer Update for Surgeons 2005 (3) |
DR ELEFTHERIOS P MAMOUNAS: John
Bryant presented data at the last St
Gallen meeting evaluating the recurrence
score and Adjuvant! Online, and
they seem to perform independently to
a certain extent. Adjuvant! Online will
add to the recurrence score, and the
recurrence will add to Adjuvant! Online.
Peter Ravdin is working with us to modify
Adjuvant! Online to introduce the
recurrence score; they provide complementary
information, which is important
for the patient. However, Adjuvant!
Online doesn’t provide any prediction on
benefit from therapy, whereas the recurrence
score adds prognostic and predictive
value.
The role of adjuvant
aromatase inhibitors in
postmenopausal women
| Breast Cancer Update 2005 (2) |
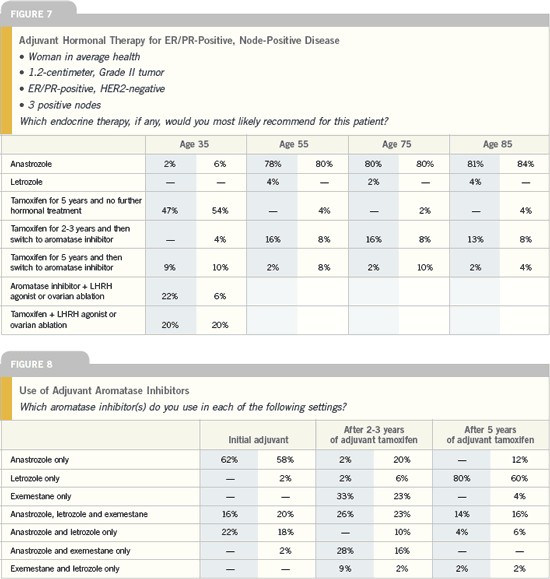
DR I CRAIG HENDERSON: Based on
data from various adjuvant endocrine
therapy trials, I believe it is unreasonab le
to withhold aromatase inhibitors from
postmenopausal women with hormone
receptor-positive disease. ATAC is still
the definitive adjuvant trial in terms of
comparing tamoxifen to an aromatase
inhibitor, and the data are very compelling.
An aromatase inhibitor is now my
drug of choice, and that changed in just
the past few years.
As for switching patients from
tamoxifen to an aromatase inhibitor, I
discuss this with every postmenopausal
patient on tamoxifen. We don’t know know the optimal duration of various
endocrine therapies. While we know
that five years of tamoxifen is as good
as or better than 10 years, the optimal
duration of aromatase inhibitors is
unknown at this time.
| Breast Cancer Update 2005 (7) |
DR ROWAN T CHLEBOWSKI: If you
start with tamoxifen, after two and a
half, three or five years, more patients
will have relapsed than on an aromatase
inhibitor. A substantial number of those
patients will be irretrievable — they
have incurable disease — and so you’re
banking on the fact that you’ll be able
to capture more patients later, but we
don’t have any data for that. That’s just
speculation.
While I believe that sequencing therapy
may be better, ultimately, I still don’t see any reason not to start with the most
effective therapy. An aromatase inhibitor
followed by tamoxifen or a nonsteroidal
aromatase inhibitor alone makes more
sense to me. We have to wait to see the
data from the BIG FEMTA trial, which
includes an arm with letrozole as initial
treatment followed by tamoxifen.
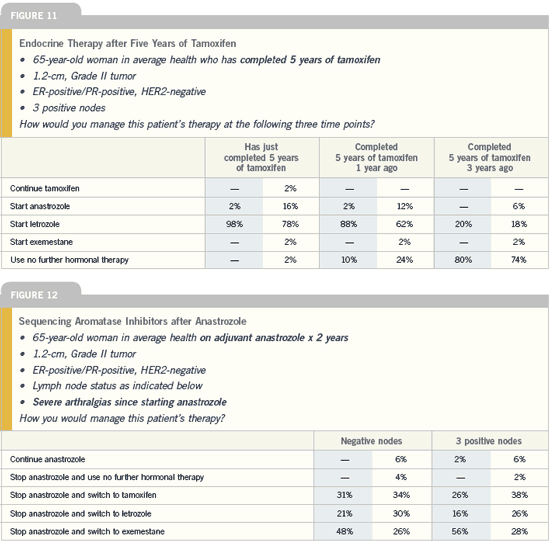
| Breast Cancer Update 2005 (5) |
DR DEBU TRIPATHY: I believe a clear,
consistent story is emerging without a
lot of conflicts and conundrums: Adjuvant
aromatase inhibitors are better
than tamoxifen. Whether the aromatase
inhibitors are used at the time of initial
diagnosis, after two to three years or
five years of tamoxifen, there is a favorable
impact on local, distant and even
contralateral breast cancer recurrences.
The unresolved questions are: Should
you use a little tamoxifen, maybe two
years and then cross over? Should you
only use an aromatase inhibitor right
off the bat and maybe even think of
continuing beyond five years? The trial
that will provide the most information
in this regard is the BIG FEMTA/BIG
1-98 trial.
| Breast Cancer Update 2005 (4) |
DR WILLIAM J GRADISHAR: I sit on
the NCCN guidelines breast cancer committee, and if you evaluate the next
rendition of the guidelines, you’ll find we
have not dismissed the use of tamoxifen
but rather moved the use of aromatase
inhibitors up front. Within the NCCN
guidelines, we’re trying to select the
aromatase inhibitor to be used based
on the design of the study. For firstline
therapy, we would use anastrozole.
If a patient has been on tamoxifen for
a period of time, exemestane is now a
legitimate choice, and after five years of
tamoxifen, letrozole is an option. We
view all of these agents as active and well
tolerated.
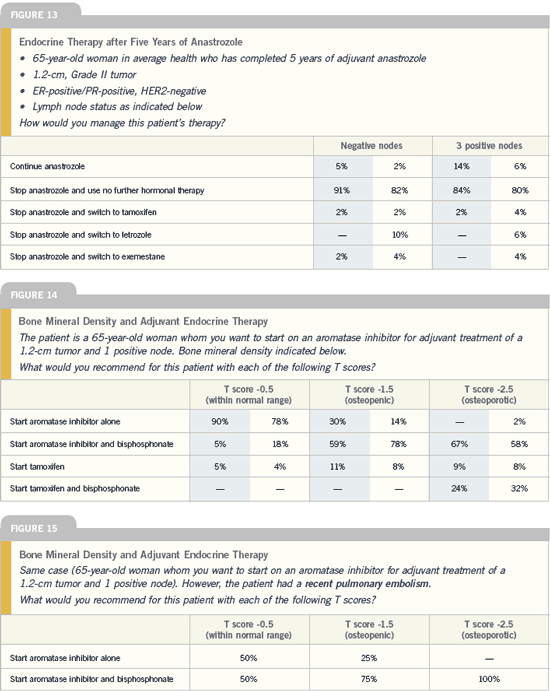
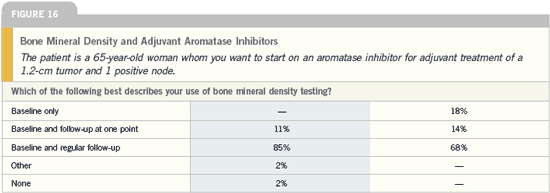
Effects of aromatase
inhibitors on bone
| Breast Cancer Update 2005 (7) |
DR CHLEBOWSKI: The five-year toxicity
data are very favorable for anastrozole
compared to tamoxifen because the three
life-threatening toxicities — endometrial
cancer, arterial and venous vascular
events — were all significantly less with
anastrozole. Many oncologists have a lot
of concern regarding bones, but I believe
it’s going to be not only a preventable,
treatable situation but also something
that is likely to go away completely in
the near future. There is no difference
in hip fractures after 68 months with
anastrozole and tamoxifen. This is for a
group of patients who had no prescreening
when they entered the study and no
ongoing protocol-defined follow-up for
bone. If you’re going to actually do any
screening, any treating, you’re going to
have lower numbers than that.
| Breast Cancer Update 2005 (1) |
DR MICHAEL BAUM: The fracture rate
incidence is becoming a little more reassuring.
An excess fracture rate occurs
in the first two or three years, but then
the lines are beginning to come together.
As patients stop taking anastrozole,
the fracture rate returns to that of the
patients randomly assigned to tamoxifen.
Furthermore, so far, no difference has
occurred in fractures of the neck or
femur, which are of particular concern.
I think the issue of bone is easy to
manage. We should be alert to it, monitor
bone mineral density, perhaps exclude
patients who have established osteoporosis,
and then be ready to intervene
with a bisphosphonate when the patient
becomes osteopenic.
Aromatase inhibitors
and arthralgias
| Lynn Sage Breast Cancer Symposium 2005 |
In the Arimidex or Tamoxifen Alone or
in Combination (ATAC) trial, arthralgia
was reported by 35.6% vs 29.4% of
patients on anastrozole and tamoxifen,
respectively, at the 68-month followup.
While the incidence was significantly
higher in the anastrozole arm
(p < 0.0001), it is noteworthy that the
incidence of arthralgia with tamoxifen
was also high (29.4%), with an absolute
difference of 6.2%. Most arthralgia
incidences occurred early in both treatment
arms (75% in the first 33 months)
and were primarily mild to moderate in
intensity.
— Paul Plourde et al. Poster. Lynn Sage
Breast Cancer Symposium 2005.
| Breast Cancer Update 2005 (4) |
DR ANTHONY HOWELL: Matt Ellis’
group presented an interesting abstract
at San Antonio indicating that women
with these joint symptoms may have
lowered vitamin D levels and that giving
them vitamin D improved some of the
joint symptoms. The data are very early,
and they are conducting more studies,
but if we could solve this joint problem
with vitamin D it would be extraordinary.
We know from the ATAC trial that
more serious adverse events are associated
with tamoxifen than with anastrozole
and that despite the joint symptoms,
patients tend to stay on anastrozole more
than they stay on tamoxifen, which is an
important efficacy issue.
Adjuvant endocrine therapy
in premenopausal women
| Patterns of Care 2004 (2) |
DR JOYCE O’SHAUGHNESSY: I have
combined an LHRH agonist with an
aromatase inhibitor in premenopausal
women, but it’s rare because for women
who are at high enough risk for that
therapy — multiple positive nodes or
even node-positive, HER2-positive
breast cancer — I generally recommend oophorectomy, and then I’m comfortable
with an aromatase inhibitor.
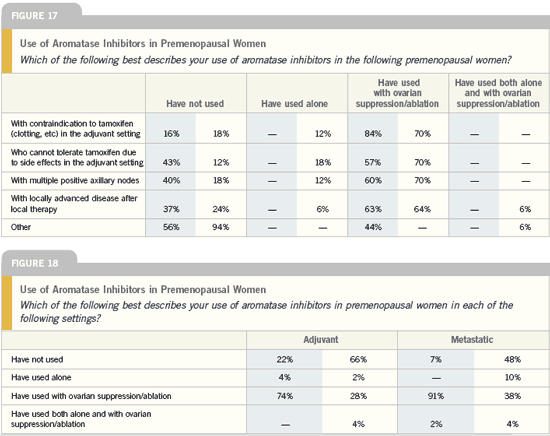
| Meet The Professors 2005 (3) |
DR ROBERT W CARLSON: The data
today are quite convincing that the
aromatase inhibitors should play a role
as adjuvant hormonal therapy for postmenopausal
women with ER-positive
breast cancer. Precisely how to sequence
or incorporate those data into the
premenopausal subset is much less clear.
We do know that the aromatase inhibitors
do not suppress circulating estrogen
levels adequately in women with functioning
ovaries, whether or not they have
menstrual function. Therefore, if you’re
going to use an AI for a young woman,
you have to be certain that she is postmenopausal,
or I think she should be
enrolled in one of the prospective trials
evaluating the use of ovarian suppression
and an AI in premenopausal women.
We do know that a number of women
stop having menstrual function or periods
subsequent to cytotoxic chemotherapy,
yet their ovaries continue to cycle.
A substantial proportion of women
also stop having ovarian function with
cytotoxic chemotherapy, at least over
the short term, but on further follow-up,
their ovarian function returns.
| ASCO 2004 Technology Report |
Cessation of menses does not necessarily
mean absence of ovarian function, as
premenopausal estradiol levels may be
found in women experiencing chemotherapy-
related amenorrhea. There is
widespread agreement that aromatase
inhibitors should not be employed as
monotherapy in premenopausal women.
This view stems from the lack of evidence
for adequate estrogen suppression and
potential for stimulation of the ovaries
via increased gonadotropin release.
— Eric P Winer, MD et al.
J Clin Oncol 2005;23(3):619-29.
| Breast Cancer Update 2005 (4) |
DR MICHAEL GNANT: We were particularly
interested in younger patients
because they are physiologically used to
higher levels of estrogen from their functioning
ovaries. We undertook ABCSG-
12 to first establish the severity of that
treatment-induced bone loss and, second, to see whether it can be prevented or
treated. We found out that a significant
loss occurs — on average close to
15 percent — in these premenopausal
women treated with endocrine therapy
with goserelin and with tamoxifen or
anastrozole. We also discovered that it
could be prevented with zoledronic acid
given twice a year.
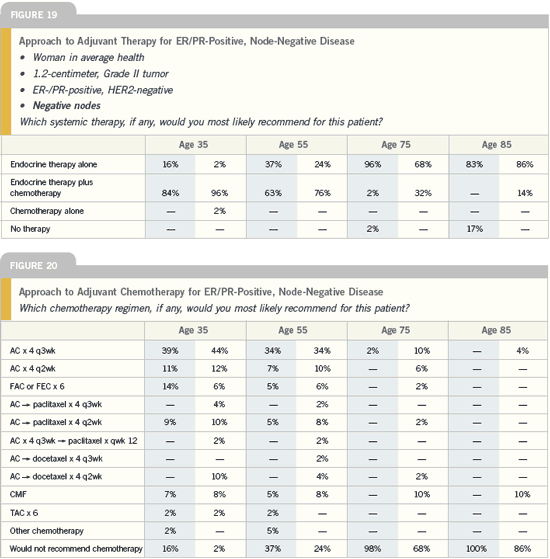
| Breast Cancer Update 2005 (4) |
DR HOWELL: Three important randomized
trials are enrolling premenopausal
women with hormone-receptive disease
— SOFT, TEXT and PERCHE. The
ABCSG-AU12 trial randomly assigned
approximately 2,000 patients to goserelin
plus tamoxifen versus goserelin plus
anastrozole, with a second randomization
to zoledronic acid or not. That
study will tell us whether tamoxifen or
an aromatase inhibitor is superior when
combined with goserelin in premenopausal
women. We expect that goserelin
with anastrozole will be better, which is
why so many patients are already being
treated off protocol.
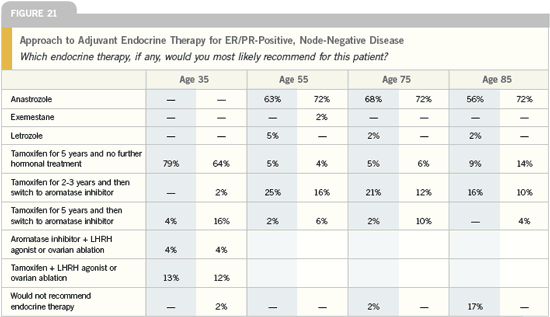
Sequencing aromatase inhibitors
after adjuvant tamoxifen
| Breast Cancer Update 2005 (2) |
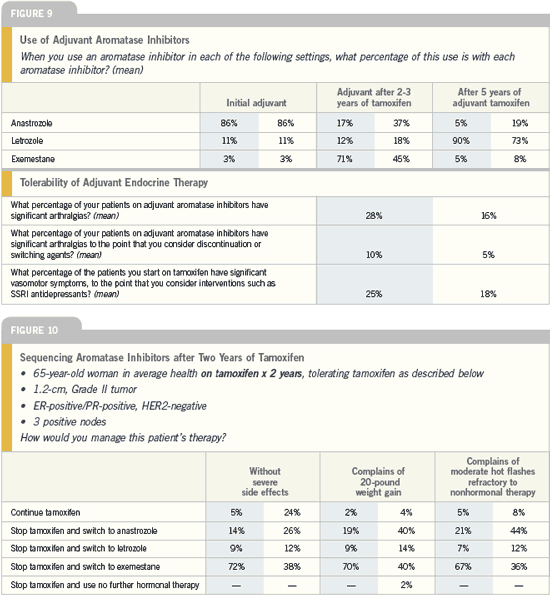
DR BAUM: I am now absolutely confident
that women who have been on tamoxifen
for two or three years should switch
to an aromatase inhibitor. We have
excellent data for both exemestane and
anastrozole from three trials. Boccardo’s
small ITA trial with anastrozole was
the first to report, followed by the large
IES study with exemestane and the joint
Austrian-German study of anastrozole
presented in San Antonio. Overwhelming
evidence indicates that a switch to an
aromatase inhibitor is beneficial.
I recommend the switch regardless
of whether the patient has been on
tamoxifen for one year or four years. You
can wait forever for refinements, but no
one is ever going to do a trial of a switch
at one year or a switch at four years. We
just have to stretch the available evidence
and be sensible about it, and I think it
would be reasonab le to switch.
The MA17 trial is a well-conducted
trial in women who have already received
five years of tamoxifen. It shows proof of
the principle that you can influence the
natural history of breast cancer after five
years of tamoxifen.
| Breast Cancer Update 2005 (2) |
DR MAURA N DICKLER: The aromatase
inhibitors add benefit immediately
after surgery, after two to three years
of tamoxifen or as extended adjuvant
therapy. In breast cancer, the highest
risk of recurrence is typically within the
first two to three years after surgery. In
women who participated in the ATAC
trial, you can see a difference in the
disease-free survival curves well before
the two and a half year mark. Not only do
you lose patients to an early breast cancer
recurrence in the first two to three years,
but you also lose some women to adverse
events on the tamoxifen arm.
The IES study and MA17 do not
really take those facts into consideration
because those patients have already
dropped out prior to randomization. I
typically offer anastrozole to the majority
of postmenopausal patients with
receptor-positive tumors after surgery
and chemotherapy. When patients come
in after two to three years of tamoxifen,
I discuss switching them to an aromatase
inhibitor. At the end of five years of
tamoxifen, I discuss letrozole.
| Breast Cancer Update 2005 (4) |
DR HOWELL: I use exemestane after
two to three years of tamoxifen based on
the IES data. However, if you compare
the IES exemestane data to the data
from the combined ARNO 95 and
ABCSG-8 trials, in which the patients
were switched to anastrozole, the agents
appear to be similar in terms of efficacy.
The hazard ratio for relapse-free survival
was 0.73 in the IES study and 0.60 in
the ARNO study, so I believe these two
agents are equivalent in this situation.
We now have data to support the use
of either anastrozole or exemestane after
two or three years of tamoxifen. After
five years of tamoxifen, we have only the
MA17 trial data, so I use letrozole in
this setting.
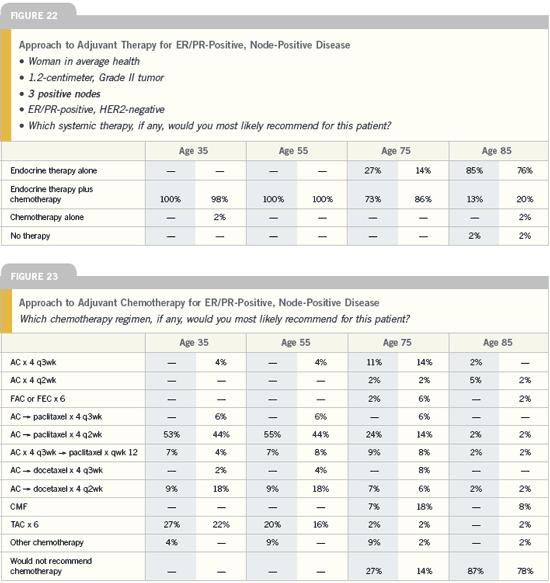
| Breast Cancer Update 2005 (3) |
DR RAIMUND V JAKESZ: In the combined trials of ABCSG-8 and
ARNO 95, more than 3,200 postmenopausal
patients, all with receptor-positive
disease, were exposed to two years
of adjuvant tamoxifen after surgery.
We then randomly assigned them to
tamoxifen or anastrozole for three years.
The data were clean and informative.
In the IES trial, exemestane resulted
in a risk reduction of approximately 35
percent, whereas in the combined trials
the risk of an event was reduced by 40
percent with anastrozole. Most of the
difference in event rate with anastrozole
was due to a huge reduction in distant
metastases.
| Breast Cancer Update 2005
(Special CME Meeting Edition) |
DR C KENT OSBORNE: Several groups
have looked at statistical modeling of the optimal long-term sequencing of an AI
after tamoxifen versus immediate use of
an AI — Jack Cuzick’s group in London,
the Dana-Farber group with Hal Burstein,
and our own group in Houston with
our statistician, Sue Hilsenbeck. All of
these models suggested similar findings,
and they could not rule out a moderate
benefit from sequencing compared to
immediate use if one looks at the longterm
results after 10 years in the large
subgroup of ER/PR-positive tumors.
Although there is a peak in recurrence
at two to three years, ultimately more
patients recur after year five than in
the first five years, and the sequence of
tamoxifen followed by an AI could turn
out to be a better strategy. While it is
true that we can’t necessarily go by the
results of mathematical models, they do
provide some evidence of what the possibilities
of these different strategies might
be over the long term.
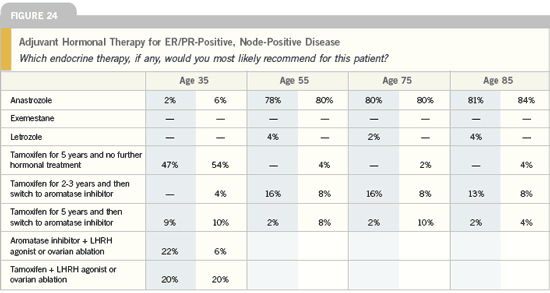
| Breast Cancer Update 2005 (9) |
DR MAMOUNAS: It is important to study
the duration of aromatase inhibitor therapy.
The NSABP will take patients
who complete five years of an aromatase
inhibitor or took tamoxifen for two to
three years and then switched to an
aromatase inhibitor and randomly assign
them to either continue an aromatase
inhibitor — letrozole — versus placebo
for five years. We will essentially do what
we did in the NSABP-B-14 extension
trial but with aromatase inhibitors.
68-month follow-up of the ATAC Trial
The present data suggest that it is not
appropriate to wait five years to start an
aromatase inhibitor. Furthermore, the
higher rates of recurrence (especially in
years 1–3) and the increased numbers
of adverse events and treatment withdrawals
associated with tamoxifen lend
support to the approach of offering the
most effective and well-tolerated therapy
at the earliest opportunity. Five years of
anastrozole should now be considered as
the preferred initial adjuvant endocrine
treatment for postmenopausal women
with hormone receptor-positive localized
breast cancer.
— ATAC Trialists’ Group.
Lancet 2005;365(9453):60-2.
| Breast Cancer Update 2005 (1) |
DR BAUM: The ATAC trial has reached
an important point in its evolution with a
median follow-up of 68 months. Almost
all of the patients are now off therapy,
and we have one year of follow-up after
the therapy was completed. I believe
this is probably the most important of
the three analyses, and this latest analysis
allows me, as a practicing clinician,
to change my mind and my practice. I
now would say that anastrozole is the
preferred initial treatment for postmenopausal
women with hormone receptorpositive
disease.
The simplest interpretation of the
results is that anastrozole prevents one in
four of the relapses we see in patients on
tamoxifen. That translates into highly
significant improvements in disease-free
survival, recurrence-free survival and distant
disease-free survival.
In the hazard rate analysis plot from
the ATAC trial, we’re seeing two peaks
with tamoxifen. The first peak is lowered
with tamoxifen, but a peak still
occurs. In the anastrozole arm, the initial
peak is lost and the second peak is flatter.
I believe this is the most profoundly
important observation in this trial, not only to help make therapeutic decisions
but also to give a fascinating biological
insight. The strongest argument for
starting adjuvant endocrine therapy with
an aromatase inhibitor is that anastrozole
almost ablates that first peak. If you wait
two to three years, as some of the trials
are reporting, the effects are wonderful,
but meanwhile, you’ve lost those patients
who will relapse and ultimately die in
those first two years.
BIG FEMTA/IBCSG-1-98/BIG
1-98:
Letrozole versus tamoxifen
up front or sequentially
| Breast Cancer Update 2005 (6) |
DR JACK CUZICK: The efficacy results
in BIG FEMTA were essentially the
same as those in the ATAC trial at the
30-month point. The hazard reduction
was similar, and the side-effect profile
was, by and large, the same, although it
was reported differently. A few differences
were seen. They found a benefit for
letrozole only in patients with node-positive
disease, which is difficult to understand.
It’s probably a chance finding, but
we need to follow that.
At this stage, they’ve found no difference
in efficacy between the patients with
PR-positive and PR-negative disease. We
have to acknowledge that the data are
different from what’s been observed in
other trials.
The third and most worrying finding
is the substantial excess in cardiovascular
deaths for letrozole compared to
tamoxifen, which hasn’t been observed
in the trials with anastrozole. Whether
this is due to chance or differences in
cardiovascular mortality is important to
know. Letrozole is a slightly more potent
aromatase inhibitor, and it is not clear
whether that has an impact.
| Breast Cancer Update 2005 (5) |
J MICHAEL DIXON: The BIG 1-98 data
are a short-term analysis — follow-up
is only 25.8 months, whereas for the
ATAC trial the follow-up is five years
— and some concerns exist as to how
the BIG 1-98 data are being analyzed.
The trial has four arms, and patients
who switched therapy after two years
were included in the analysis, but only up
until the time when they were switched.
That’s a bit unusual because one would
expect some of the benefit from the first
two years of tamoxifen and letrozole to
continue.

BCIRG 001: Adjuvant
TAC versus TAC
| Breast Cancer Update 2005 (1) |
DR JOHN R MACKEY: In our first study,
BCIRG 001, 1,500 women from 21
countries were randomly assigned to six cycles of adjuvant TAC or FAC. The
women enrolled in the trial had nodepositive
disease. We now have mature
results with five years of follow-up.
The trial demonstrated that adjuvant
TAC significantly improved disease-free
survival by 28 percent in relative terms
(p = 0.001). Overall survival was also
strikingly improved; the trial demonstrated
a 30 percent relative reduction in
mortality with adjuvant TAC, which was
an absolute six percent improvement in
overall survival.
This would be a perfect story if an
increase in side effects did not occur. In
fact, TAC was associated with a high rate
of febrile neutropenia. Approximately 25
percent of the women receiving TAC
experienced an episode of febrile neutropenia,
which was not unexpected because
primary prophylaxis with G-CSF was
not allowed. We now know that if we
were to do the study again and administer
TAC with G-CSF, we would see a
febrile neutropenia rate, on a per-patient
basis, of about three to six percent.
NSABP trial B-38
| Breast Cancer Update 2004 (3) |
DR CHARLES E GEYER JR: Two key
adjuvant trials have been BCIRG 001,
evaluating TAC versus FAC, and the
CALGB dose-dense trial 9741 of AC
paclitaxel. Currently, our view is that
TAC appears to be the optimal way to
administer an anthracycline/docetaxel
regimen, and dose-dense AC/paclitaxel
is the optimal way to administer those
agents.
Which is better? It’s impossible to
answer that question without performing
a clinical trial, which is why we developed
trial NSABP-B-38. It’s a pragmatic
design in which we regard TAC as our
control arm. A clear advantage of dosedense
therapy is that it is so well tolerated,
and it clearly affords the opportunity
to add a fourth drug to the paclitaxel.
TAC is a maximally tolerated regimen.
You really can’t push it much more, so
we sought a candidate drug to combine
with paclitaxel.
Adjuvant clinical trials
incorporating capecitabine
| Breast Cancer Update 2005 (3) |
DR O’SHAUGHNESSY: The vinorelbine/
capecitabine combination is one of
numerous capecitabine combinations
being evaluated in European adjuvant
trials. I’m not aware of any adjuvant
or neoadjuvant studies evaluating capecitabine/
paclitaxel; however, a number
of neoadjuvant and adjuvant trials
are evaluating capecitabine/docetaxel.
Even if I had data with capecitabine/
paclitaxel, I probably would not have
considered evaluating that combination — as opposed to capecitabine/docetaxel
— in our adjuvant trial. In metastatic
disease, docetaxel 75 mg/m2 in combination
with capecitabine has a clear survival
advantage compared to docetaxel
100 mg/m2. Usually, we try to take that
advantage in survival in metastatic disease
and immediately move it into the
adjuvant setting.
Inclusion of older patients in
trials of adjuvant chemotherapy
Our study adds to the increasing number
of trials that suggest that older patients
in fair to good health tolerate standard
chemotherapy regimens, and even more
intensive regimens, almost as well as
younger patients. Moreover, and more
importantly, this study suggests that the
added value gained from more intensive
chemotherapy regimens commonly used
in the adjuvant setting might be shared
by older patients and not limited to
younger age groups.
— Hyman B Muss, MD et al.
JAMA 2005;293(9):1073-81.
Select publications
|

