| |
Hormonal Therapy for Metastatic Disease |
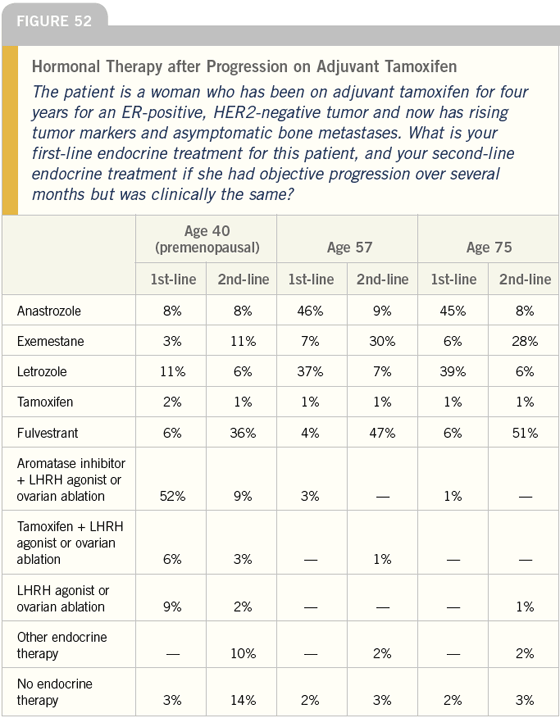
DR LOVE: Bob, how do you approach women with ER-positive disease who relapse while on adjuvant tamoxifen (Figure 52)?
DR CARLSON: This case shows us that we have a tremendous educational opportunity. Over 20 percent of oncologists are recommending one of the aromatase inhibitors without ovarian ablation to a 40-year-old premenopausal woman.
This suggests that at least one in five women in this situation is being offered an inactive first-line or second-line agent.
I think these data are probably accurate and reflect an important problem. In my practice, I would offer such a woman enrollment in a clinical trial in which we are looking to discover the benefit of goserelin plus anastrozole in young premenopausal women.
We initiated this trial because we realized many people were using this approach despite a paucity of data about it. We will have a poster at the 2004 San Antonio Breast Cancer Symposium reporting on the first 22 or 23 women enrolled in the trial.
DR LOVE: How many responses were seen?
DR CARLSON: The data is embargoed, but I can tell you that it is the highest response rate to hormonal therapy that I have ever seen.
DR LOVE: Interesting. What do you tend to do in these three situations in a nonprotocol setting?
DR CARLSON: For a premenopausal woman, I typically use ovarian ablation and add an aromatase inhibitor. I do not use ovarian suppression because I expect to keep this woman on hormonal therapies for a long time.
The most active hormonal therapies we have are either active only in postmenopausal women or have been studied primarily in postmenopausal women. If a woman is functionally postmenopausal, decision-making is easier and more options are available. I also believe that most women are likely to tolerate a one-shot laparoscopic oophorectomy better than years of monthly LHRH agonist injections.
In a postmenopausal woman, I present an aromatase inhibitor and fulvestrant as options. I go over the data comparing anastrozole and fulvestrant and explain that perhaps fulvestrant offers a slight improvement in duration of clinical benefit, but that no known differences exist with regard to survival and few with regard to quality of life.
Probably one third of these women will choose fulvestrant and the others will select an aromatase inhibitor. I use anastrozole as my first-line aromatase inhibitor, but I actually believe that they are all created equal in terms of antitumor efficacy and toxicity. I tend to always use the same one so I won’t get confused. That is truly the only reason I prefer one instead of the other.
DR LOVE: It’s interesting that you present both options and about one third of your patients select fulvestrant. A more typical answer I hear from oncologists is, “I present an aromatase inhibitor because it is more convenient.”
DR CARLSON: I think it is a question of for whom it is convenient and why. It relates back to the issue of chemotherapy and choosing toxicity. I always talk to women and ask, “Which of these toxicities is least concerning to you?”
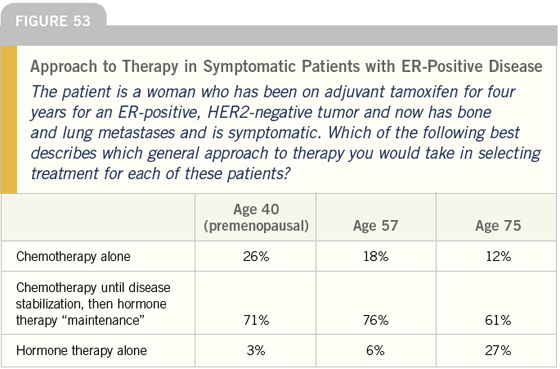
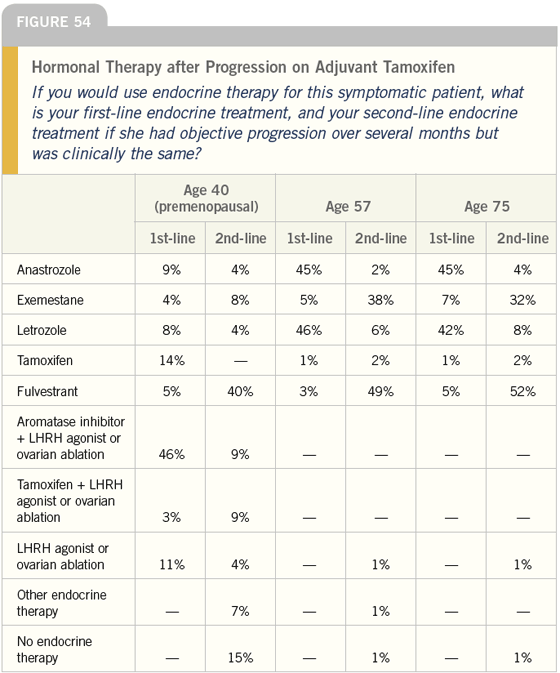
DR LOVE: The most common strategy we see for the symptomatic patient with ER-positive disease is initiating chemotherapy until the patient is stabilized, and then switching to hormonal maintenance (Figures 53 and 54). Is that a strategy you use?
DR CARLSON: For a symptomatic patient, starting with chemotherapy is a reasonable strategy, and one that I would use with this type of patient.
Hopefully, if the patient does well and becomes substantially less symptomatic due to response, the issue of toxicity will become much more important. Switching her to hormonal therapy at that point in time is reasonable. I agree fully with the majority of respondents with regard to this question.
DR LOVE: Joyce, for a patient who is gravely ill with a high tumor burden, do you use the strategy of chemotherapy to induce a remission and then maintenance hormones?
DR O’SHAUGHNESSY: I just did that with a patient, but I do not do it often. This particular woman was young, premenopausal and presented with a heavy liver burden and bone disease.
She also had some adenopathy in her axilla. I gave her six cycles of TAC and she had a good response.
I knew I could not give her TAC forever, so I stopped it. She is postmenopausal now and is on letrozole. I am watching her estradiol closely. I use this strategy only for the sickest of patients and it is not common.
DR LOVE: Bob, you chair the NCCN Breast Committee. What do they say about this?
DR CARLSON: The practice of initially treating patients who have ER-positive breast cancer with chemotherapy and then switching to hormonal therapy is not addressed in the NCCN guidelines; however, it’s a common strategy that makes sense.
In general, the NCCN guidelines classify women into two groups: those who should be given endocrine therapy until they sequence through all of them or develop organ impairment, and those who should be given chemotherapy until they have exhausted all of the reasonable chemotherapy options.
The guidelines do, however, recommend that women who have substantial organ dysfunction — even those with hormone receptor-positive disease — be treated initially with cytotoxic chemotherapy.
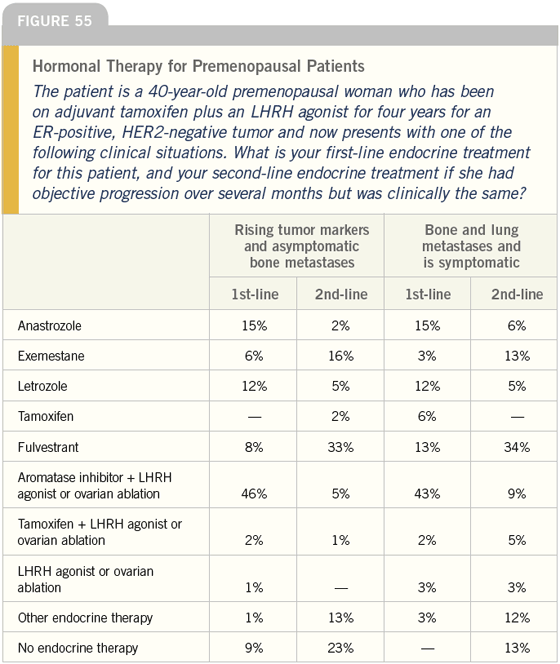
DR LOVE: We often see premenopausal patients such as these who experience relapse on adjuvant tamoxifen. Joyce, I am curious about how you generally approach a patient with ER-positive, HER2-negative disease who has been on tamoxifen for two or three years and develops metastases?
DR O’SHAUGHNESSY: In general, I think we should treat those patients with hormonal therapy. They have been on tamoxifen for two or three years. That is not a terribly long disease-free interval but it is not bad either. I would consider hormonal therapy unless the patient was approaching or in visceral crisis. If the patient was not horrendously tumorburdened and horribly symptomatic and postmenopausal, then I would favor hormonal therapy and go right on to an aromatase inhibitor.
In premenopausal patients (Figure 55), I have had a phenomenal success rate with LHRH analogs or oophorectomy along with an aromatase inhibitor. I personally find that premenopausal women benefit from oophorectomy followed by an aromatase inhibitor and that is what I do first line.
DR LOVE: What is your usual secondline therapy for patients who progress on the aromatase inhibitor?
DR O’SHAUGHNESSY: I either use exemestane or tamoxifen — depending on when (or if) the patient last had tamoxifen. In a woman who progressed after only two or three years on tamoxifen, I would not use tamoxifen again, but if she had tamoxifen many years ago, I would probably go back to it.
I also use fulvestrant but I do not use it often right after an aromatase inhibitor. Neil, we all come up with our own hunches on things, and the good responses to fulvestrant that I have seen have not been immediately following an aromatase inhibitor. They have been in postmenopausal women whose bodies have come back to homeostasis, if you will, with regard to their estrogen levels.
I’ll give you an example. I have this remarkable patient with metastatic disease whom I met about five years ago. She was 65 years old at the time and had been on tamoxifen for 20 years for metastatic bone disease.
DR LOVE: Wow.
DR O’SHAUGHNESSY: Twenty years! She was Steve Jones’ patient and I inherited her. The cancer progressed in her bones so I initiated anastrozole and she did well for about two and a half to three years. Then she became symptomatic and crippled with bone pain. Fulvestrant was not yet available.
She was chemotherapy-naïve so I started her on vinorelbine. She had a good response but after a while had difficulty handling the toxicity of the chemotherapy. We stopped it and she had a nice remission for nine months.
She then progressed again, so I started her on fulvestrant and she has been in a remission for almost two years. I am so pleased with how well she has done, and maybe she would have done just as well on fulvestrant if I had brought it in after an aromatase inhibitor, but I don’t have any similar success stories to report after an aromatase inhibitor.
We all try to strategize about our hormonal therapies to figure out when to introduce these truly important agents at the most opportune time. That is why we call it “practice.” But I am trying to use fulvestrant away from an aromatase inhibitor to let the body return to homeostasis.
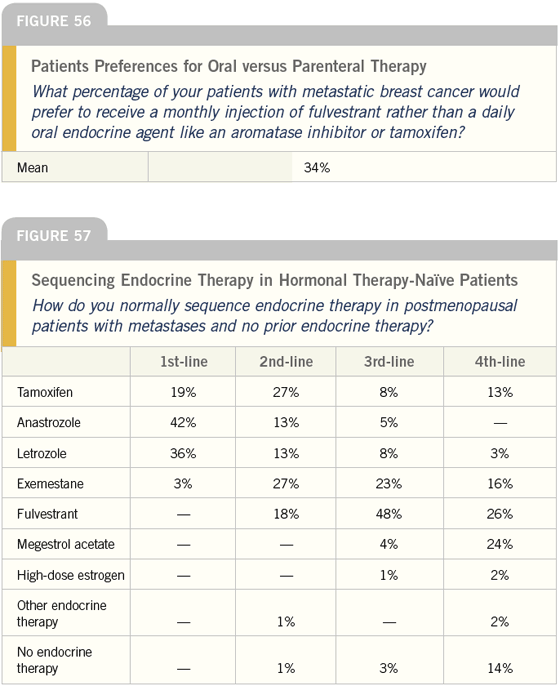
DR LOVE: Joyce, if we could speak for a moment about how these agents are administered, what percentage of your patients with metastatic breast cancer do you believe would rather receive a monthly injection of a hormonal agent, such as fulvestrant, versus taking a pill daily, such as an aromatase inhibitor or tamoxifen (Figure 56)?
DR O’SHAUGHNESSY: I believe approximately 30 percent of my patients would prefer to receive the injection. I find that maybe a third of my patients struggle with insurance coverage for oral medications, so they would prefer an injection.
DR LOVE: We have found that when posing this question to oncologists, oncology nurses and patients, they consistently answer between 30 to 40 percent. We also did a telephone survey of 260 patients with metastatic breast cancer, and about a third preferred parenteral therapy.
In asking patients why they would prefer an injection, we were unable to establish a clear profile — some find it more convenient, some believe injections are more effective and some just don’t like taking pills or have a problem remembering them. However, it appears physicians assume patients would prefer an oral medication because of the convenience and may not present the injection option, yet a third of patients prefer it.
DR O’SHAUGHNESSY: That’s interesting. My endocrine therapy of choice in a patient progressing on tamoxifen is an aromatase inhibitor because of the vast data available. All three of the aromatase inhibitors look great after tamoxifen, and while we have the fulvestrant versus anastrozole data, that’s only one trial.
DR LOVE: How do you feel about the responses regarding sequencing endocrine therapy (Figure 57)?
DR O’SHAUGHNESSY: Most of the clinicians chose an aromatase inhibitor up front and the data suggest that is probably optimal.
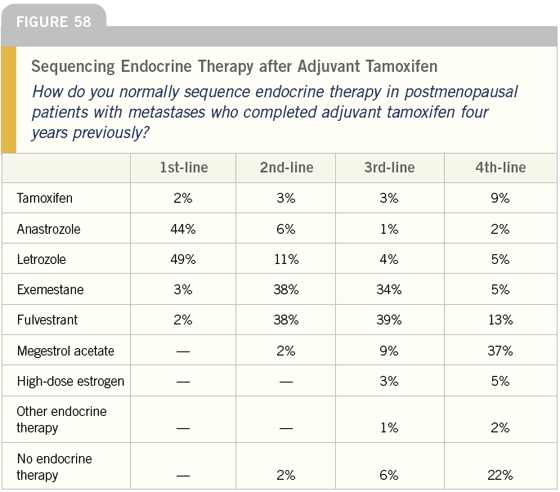
DR LOVE: What do you use after the aromatase inhibitor?
DR O’SHAUGHNESSY: I use tamoxifen or exemestane, depending on what the patient has had before, and for the next line of therapy, I use fulvestrant.
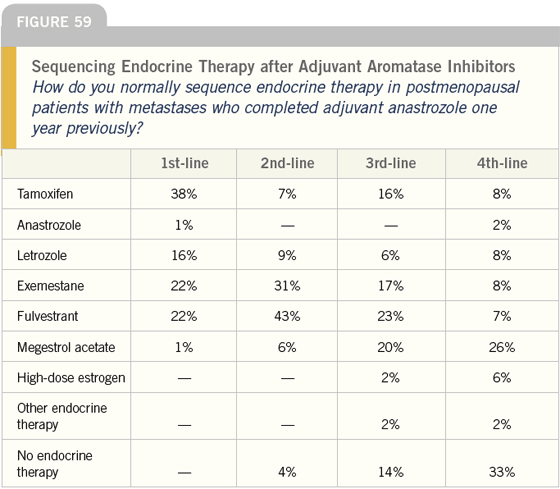
DR LOVE: Would you treat this patient who received adjuvant tamoxifen the same way (Figure 58)?
DR O’SHAUGHNESSY: I would use a nonsteroidal aromatase inhibitor, and after that I would be inclined to use exemestane. I’m not using fulvestrant after aromatase inhibitors, even though some small studies show efficacy — I just haven’t seen good responses from that sequence.
In select patients who have a relatively small tumor burden, I consider highdose estrogen therapy, particularly after the aromatase inhibitors. If the patient is heavily burdened with tumor, I select a gentle chemotherapeutic agent, my first choice being capecitabine.
DR LOVE: And how would you treat the patient who had taken adjuvant anastrozole (Figure 59)?
DR O’SHAUGHNESSY: In a patient who completed five years of an aromatase inhibitor, I would use tamoxifen. After tamoxifen, I’d probably use exemestane or fulvestrant.
DR LOVE: Bob, what are your thoughts about the sequencing of endocrine therapy in metastatic disease, specifically with regard to response to fulvestrant?
DR CARLSON: Women with breast cancer who fail on tamoxifen can clearly respond to fulvestrant, and the rate of response is equivalent to that seen with anastrozole. Also, in women with disease that has failed anastrozole who then cross over to fulvestrant, the rate of clinical benefit is substantial and in the range of about 40 percent. Patients who cross over from fulvestrant to aromatase inhibitors also show response rates around 40 percent.
Surprisingly, the magnitude of benefit from fulvestrant does not predict whether the cancer will respond to a subsequent hormonal maneuver. One rule of thumb in the past has been that the magnitude and duration of response to the most recent hormonal therapy predicts the likelihood of response for subsequent hormonal therapies. A small retrospective study suggests that may not be the case with fulvestrant.
In addition, an increasing body of preclinical evidence suggests that breast cancer that becomes resistant to tamoxifen or fulvestrant has upregulation of epidermal growth factor receptor (EGFR) and HER2 expression. As those endocrine-sensitive cells become endocrine-resistant and the EGFR and HER2 upregulate, some of the sensitivity to the endocrine agents may return if those cells are exposed to EGFR inhibitors.
Series of trials are being conducted to evaluate the role of fulvestrant or other hormonal agents in combination with gefitinib. ECOG is conducting a Phase II randomized trial comparing fulvestrant/ gefitinib to anastrozole/gefitinib.
Select publications
|

