|
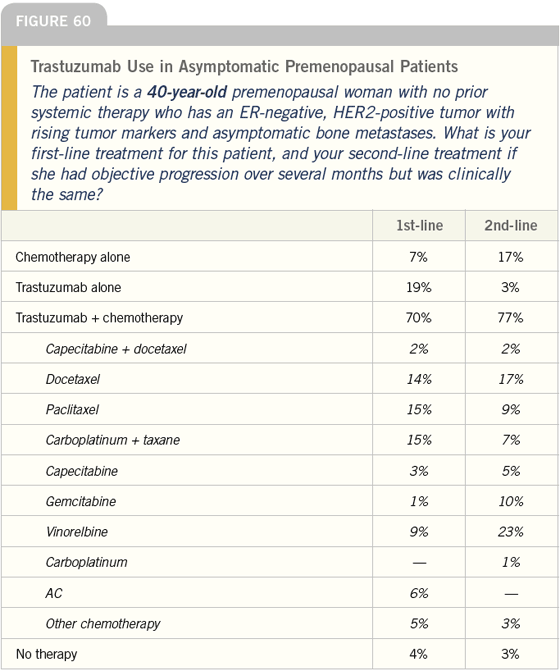

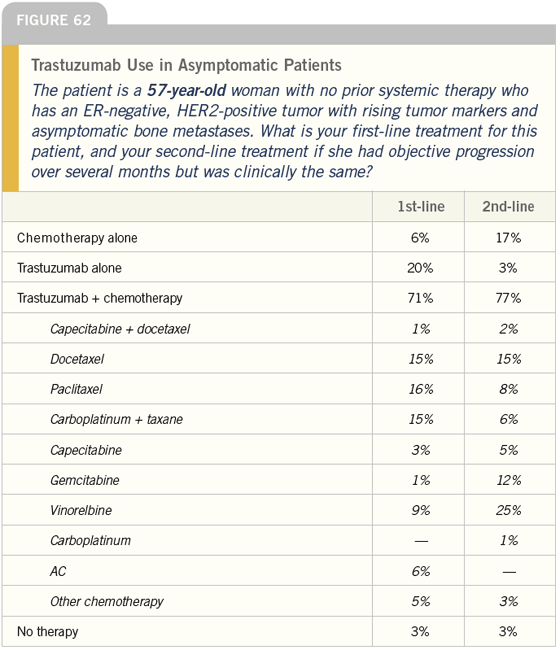
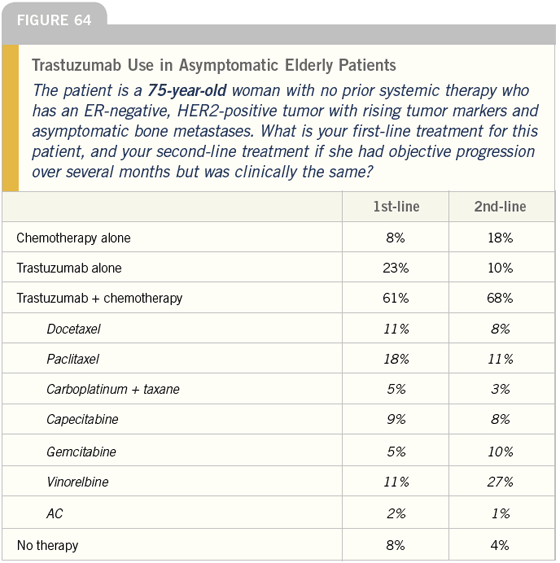
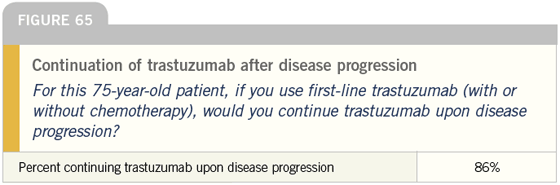
DR LOVE: Bob, how do you typically manage asymptomatic patients with ER-negative, HER2-positive tumors (Figures 60-65)?
DR CARLSON: For a younger woman, I would use trastuzumab and would discuss with her whether or not to add a taxane — most likely paclitaxel. For a 75-year-old woman, I would typically use trastuzumab as a single agent.
DR LOVE: For how long do you continue the paclitaxel?
DR CARLSON: That is a tough decision to make. I consider the magnitude and rapidity of response and toxicity experience. The more impressive the response or the less tolerable the regimen, the more inclined I am to stop the taxane sooner rather than later; however, I try to give everyone at least six months of therapy before I stop the cytotoxic part of the regimen.
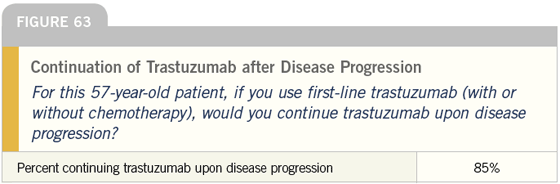
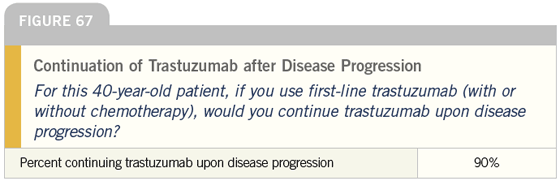
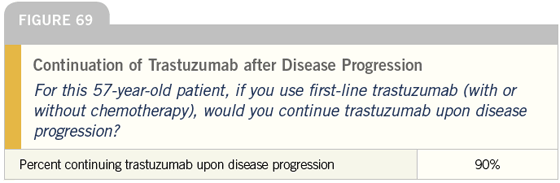
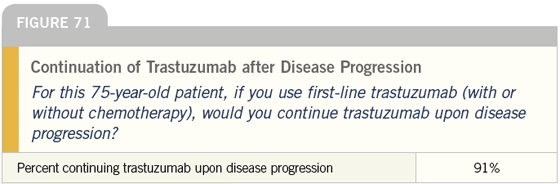
DR LOVE: It is interesting that almost all of these docs continue trastuzumab when the patient progresses to secondline therapy. Is that what you do?
DR CARLSON: In the absence of data, that is what I do. Some of that is West Coast bias, but patients are influential in this regard. Many of them, correctly or incorrectly, believe that once trastuzumab is started, it should never be stopped.
At times, we find ourselves encouraging patients to stop, and they just tell us “No.” There is a very powerful patient network in this area, and in this population I tend to continue trastuzumab indefinitely.
DR LOVE: Let me take a step back here for a moment to talk about HER2 testing. Joyce, when you are considering trastuzumab in any setting, how do you approach HER2 testing?
DR O’SHAUGHNESSY: We routinely order IHC on pathology specimens. For patients with metastatic breast cancer, if the IHC is 3+ I do not generally follow up with FISH, provided the tumor stains 3+ in 75 to 100 percent of cells.
I also determine whether the cancer is high grade and has a high proliferative fraction. Particularly in those cases, I do not order FISH for metastatic disease.
I order a FISH assay when I use adjuvant trastuzumab because in that setting I want documentation that the patient has FISH-positive disease. I also always order a FISH if the IHC is 1+ or 2+.
DR LOVE: We had an interesting case presented for our Meet The Professors audio series. The patient’s tumor was found to be IHC 0, but the doctor decided to do a FISH and it came back positive. He started trastuzumab and the woman responded very well. Do you FISH test IHC 0 tumors?
DR O’SHAUGHNESSY: I sometimes do. I do not in the adjuvant setting when I’m trying to decide between tamoxifen and an aromatase inhibitor, but in metastatic disease, I test everybody.
I believe every patient with metastatic disease needs one FISH assay in her lifetime. I actually had a patient whose tumor was labeled FISH-negative at a very large major cancer center. She moved to Dallas and her files were delayed in the mail, so I ordered a FISH test and it came back positive. She has derived benefit from trastuzumab.
These are not perfect tests by any means, and it is worthwhile to make sure you are comfortable with the results.
DR LOVE: Wow, that is a really scary case.
DR O’SHAUGHNESSY: It is a really scary case because the test was done at a very important center. The other thing I will tell you is that one woman I treated with adjuvant trastuzumab had over 10 positive nodes, a huge amount of axillary disease and an ER/PR-negative, HER2- positive tumor (3+ by IHC). It was definitely 3+ but also FISH-negative.
I emailed Soon Paik for guidance on this because I was uncertain about what to do. He told me that in the original series, some patients fell into this category. We don’t know why, nor whether a post-translational modification exists that is not amplified at the DNA level but nonetheless results in a ton of protein.
The pivotal trials evaluated the 3+ versus the 1+ or 2+, and a benefit was shown in the 3+ population. Dr Paik believes that the bulk of our data are with IHC methodology and, therefore, it is perfectly justifiable to treat this type of patient with trastuzumab.
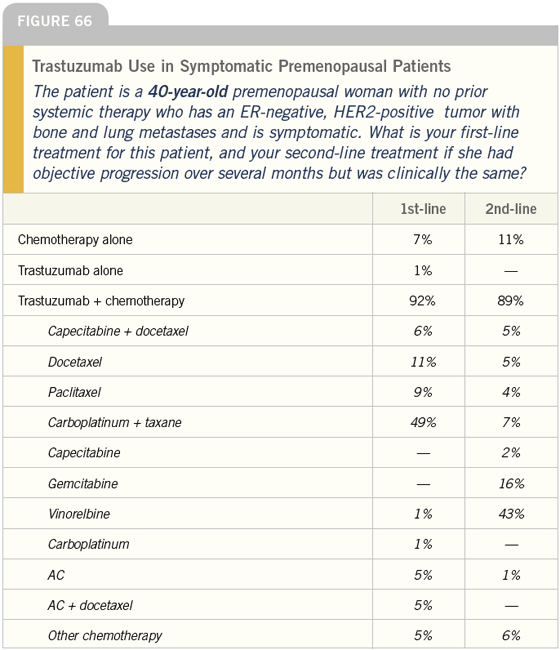
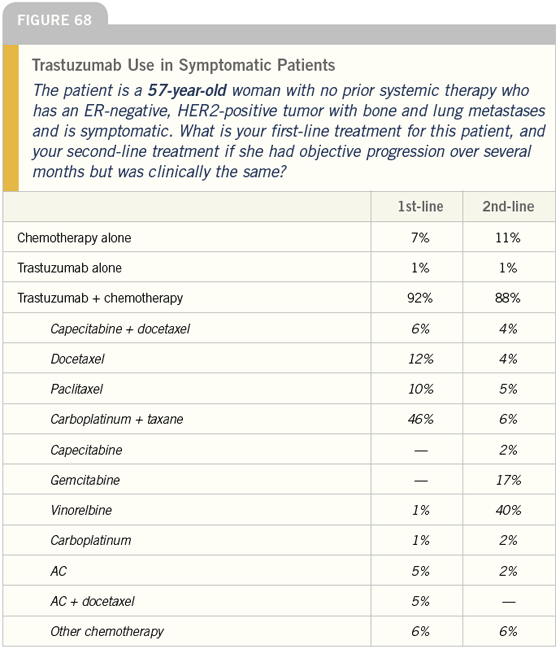
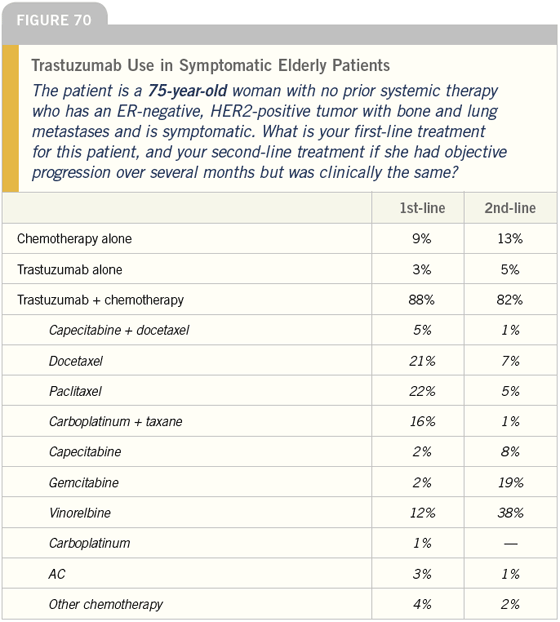
DR LOVE: Getting back to our cases, Bob, how do you treat symptomatic patients with ER-negative, HER2- positive tumors (Figures 66-71)?
DR CARLSON: In this situation I would use trastuzumab in combination with a taxane and I would consider adding carboplatin; however, in a 75-yearold woman I would be hesitant to use the triplet and would typically use trastuzumab combined with paclitaxel.
DR LOVE: We talked before about nanoparticle paclitaxel. Would you consider using that with trastuzumab?
DR CARLSON: Good question. I would certainly consider it, but I have not seen any data on that combination. One of the issues with nanoparticle paclitaxel is that its delivery mechanism is different, so it is not totally clear whether the drug equivalence to paclitaxel or docetaxel will generalize to all settings.
I would be hesitant to use it until we have prospective data suggesting that the additive or synergistic interaction of trastuzumab and the taxanes also exists for this newer agent.
DR LOVE: What would be your secondline choice after the taxane?
DR CARLSON: Vinorelbine. Approximately one fourth of patients receiving vinorelbine develop asthenia or pulmonary symptoms and have to be taken off the drug quickly, but for the rest it is a well-tolerated medication.
DR LOVE: Another issue that arises is the combination of capecitabine and trastuzumab. Will you ever use that combination?
DR CARLSON: Historically, concerns arose about incorporating a fluoropyrimidine with trastuzumab, but I think more recent data specifically evaluating capecitabine and trastuzumab suggest they are at least additive and perhaps synergistic in efficacy.
DR LOVE: When you say data, is that laboratory or clinical?
DR CARLSON: This is preclinical data in animal models that examine the issue of additive or synergistic cytotoxicity. A smattering of small Phase II trials suggest response rates at least as good as you would expect from the combination as opposed to single-agent capecitabine.
DR LOVE: What about the strategy using single-agent trastuzumab in the asymptomatic patient and then adding a chemotherapeutic agent if the patient doesn’t respond?
DR CARLSON: I have used that strategy — especially in older patients. Chuck Vogel’s data suggest that response rates, at least in the women who have FISHpositive tumors, are going to be as high as 45 to 50 percent.
DR LOVE: How do you handle cardiac monitoring for patients receiving trastuzumab?
DR CARLSON: I typically do a baseline ejection fraction and I prefer to use MUGA scans rather than ECHOs, but either is appropriate. For asymptomatic patients with good cardiac function, I typically monitor them about every six months. Drops in ejection fraction or clinical heart failure rarely occur.
DR LOVE: What do you do when a patient’s ejection fraction drops?
DR CARLSON: I stop the trastuzumab. If the patient is symptomatic, I refer her to one of my cardiology colleagues to optimize her cardiac medication. Typically, the cardiac function will improve. I have actually rechallenged a few of these patients with trastuzumab. Interestingly, the cardiac toxicity often does not reappear.
DR LOVE: Interesting. Have you seen tumor responses?
DR CARLSON: It is hard to separate whether secondary tumor responses occur, because almost all of these patients are going to be continuing antitumor therapy on an ongoing basis.
DR LOVE: Alright, we’ve talked about trastuzumab in the metastatic setting. In a moment I am going to ask you to comment on data from the adjuvant setting, but I want to ask Joyce her opinion about the MD Anderson data presented by Dr Buzdar at ASCO this year.
DR O’SHAUGHNESSY: I think it is extremely exciting and a nice step forward. I give Aman Buzdar a lot of credit for sticking with it because this was not an easy study to initiate. It took a couple of years to get off the ground because people were very concerned about combining epirubicin with trastuzumab. Aman’s solution was to lower the dose of epirubicin and keep it going for four cycles based on some work by Luca Gianni.
Luca combined doxorubicin 240 mg/m2 with trastuzumab and had a low rate of cardiac toxicity. At any rate, I think the high PCR rate of 65 percent reported from this trial indicates the anthracyclines and trastuzumab may have some important synergy.
In my practice I have been cautious about that particular combination and have not had the occasion to utilize it since the time Aman presented the data. It is something I would consider using off study if the patient was at extremely high risk with, for example, inflammatory breast cancer.
DR LOVE: What if the patient had locally advanced disease?
DR O’SHAUGHNESSY: Neil, I would consider it. I think we have to tell patients that the data is only from approximately 20 patients. Aman has more experience with this regimen than the ASCO presentation reflects, and the trastuzumab-containing arm of the trial continues to accrue. When I spoke with him recently, he had treated approximately six more patients and he is not seeing problems with the heart.
However, NSABP-B-28 reported a 4.28 percent rate of congestive heart failure with trastuzumab following AC. Out of about 20 patients, this is only 0.8 patients. In other words, he may have not yet accrued enough patients to see one congestive heart failure.
I think we have to give patients enough information so they can give good informed consent. The B-28 data are enormously helpful to doctors, and I think I would have to apply those to Aman’s data to help decide what I am going to do.
Interestingly, Frankie Holmes is the principal investigator for a preoperative trial of FEC 100 for four cycles followed by capecitabine/docetaxel (XT). Frankie would like to amend the protocol for HER2-positive patients to make it FEC 75 for four cycles followed by XT combined with trastuzumab. The goal is to gain trial experience adding trastuzumab to chemotherapy, including FEC 75, in the preoperative setting.
DR LOVE: That is fascinating. Have you used neoadjuvant trastuzumab off protocol for inflammatory and locally advanced disease?
DR O’SHAUGHNESSY: No, I have not. Usually, I use CAF preoperatively for patients with locally advanced or inflammatory disease. Then they undergo surgery and if they have residual disease, I start docetaxel/trastuzumab or paclitaxel/carboplatin/trastuzumab.
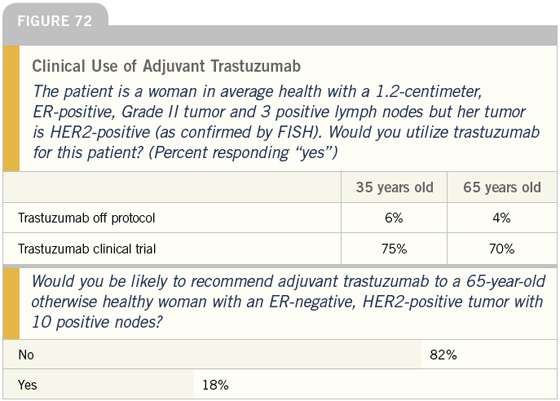
DR LOVE: Let’s talk briefly now about adjuvant trastuzumab (Figure 72). Based on the data, it seems that very few physicians would recommend trastuzumab off study for a patient with HER2-positive disease and three positive nodes, but a relatively high number would recommend a clinical trial. Do you have any thoughts about that?
DR CARLSON: I find it somewhat surprising but very reassuring that physicians are not prescribing trastuzumab off protocol without high-level evidence that the benefits exceed the long-term toxicities — especially with regard to cardiac toxicity.
DR LOVE: We have been doing these kinds of surveys for many years and it appears that physicians’ approaches to treatment are becoming much more in line with what research leaders are doing. I’m not sure why that is happening — maybe it is better education.
DR CARLSON: It might be the “Bezwoda effect” and physicians’ experiences with high-dose chemotherapy. Many community physicians took information that was a little bit disconnected, put it together and concluded that highdose therapy was superior to standard full-dose therapy. When they eventually were burned by fraudulent trial results, I think many of them paused and thought, “How many women died because of my recommendation, albeit well intentioned, about how to treat their breast cancer?”
DR LOVE: Joyce, what are your thoughts about the current clinical trials evaluating adjuvant trastuzumab?
DR O’SHAUGHNESSY: Neil, I cannot wait until we have the first data analyses. Frankly, I think that this is the most important question on the table right now. I have no doubt that it has the biggest potential impact on women and that it is the next quantum leap in terms of disease-free and overall survival in the adjuvant setting. I think it is going to have a huge impact on changing the natural history of the disease and, therefore, nothing is of greater urgency.
I am going to be a little controversial here and say I hope that after we have these adjuvant data, the breast cancer community can take a look at the future and decide whether or not we really need to wait five or six years to do very large adjuvant trials in women at high risk to be able to apply positive data from the metastatic setting.
Personally, I would probably not make a leap into the lower-risk adjuvant setting where there is a smaller benefit and greater concern about risk-to-benefit ratios; however, I think the higher-risk adjuvant setting — meaning nodepositive or locally advanced breast cancer — is the greatest ethical dilemma I have faced in my career in breast cancer.
History tells us that every substantial, definitive survival advantage in metastatic breast cancer translates into improved disease-free and overall survival in the adjuvant setting. I am not aware of any exception to that rule, and it makes sense. When treating metastatic disease, often the tumor burden can be reduced by half a log or a log. If we can impact survival with that kind of cytoreduction, we should be able to make a similar impact in the locally advanced or node-positive setting. I think this is a huge priority.
I understand that the NSABP and Intergroup may try to pool the events from their ongoing trials. Both trials have similar designs — AC followed by paclitaxel with or without trastuzumab. To my knowledge both trials are still accruing and discussions are underway to combine events, which I think would be a great contribution.
The BCIRG trial has finished accrual. We are a year to 18 months away from having enough events, but I think we may have answers a little bit earlier. Unfortunately, HER2-positive breast cancer conveys a poor prognosis and a number of events may occur in women with HER2-positive breast cancer in the control arm without trastuzumab.
DR LOVE: Based on what you are saying, for a woman at very high risk — 5 to 10 positive nodes, HER2-positive disease — do you consider nonprotocol adjuvant trastuzumab?
DR O’SHAUGHNESSY: As I mentioned, this is the issue that gives me the most grief. In the past three to four years, I have treated no more than 10 or 12 women at high risk in the adjuvant setting with trastuzumab.
They have been either patients with inflammatory breast cancer or 10 or more positive nodes. Generally, they are in the ER/PR-negative, FISH-positive group.
I treated one woman who had bulky adenopathy and eight positive nodes, and another woman with large, bulky, macrometastases, adenopathy and five positive nodes. Both of these women were young, vigorous and healthy, and had ER/PR-negative and HER2- positive disease by FISH.
I have used adjuvant trastuzumab extremely judiciously, recognizing the risks that I was taking, and I have had extremely good success. Of the 10 or 12 patients I’ve treated, only one patient has experienced relapse, and, frankly, in that case I brought the trastuzumab in late.
I gave that patient preoperative CAF followed by docetaxel without trastuzumab. She went to surgery and still had an extraordinary bulk of disease. I started vinorelbine and trastuzumab afterward and I’m afraid I was too late with the trastuzumab; she developed metastatic disease.
With the others, I started docetaxel/ trastuzumab or docetaxel/carboplatin/ trastuzumab right after the CAF or the AC and none of those patients has experienced relapse.
Select publications
|

