|
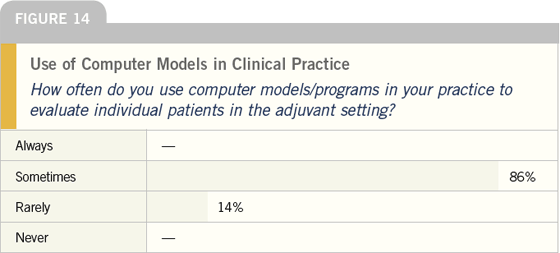
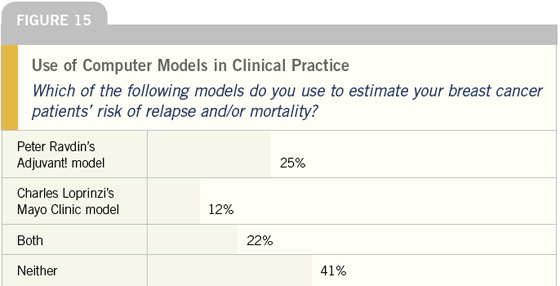
DR LOVE: The use of Peter Ravdin’s
Adjuvant! program and some of the
other computer models is a recent
phenomenon. Interestingly, it now
looks like more than half of all oncologists
either have used or are using these
models. Joyce, do you use a computer
model in making clinical decisions
(Figures 14-16)?
DR O’SHAUGHNESSY: I sometimes
use the Ravdin model. I’m surprised
to see that over half of the oncologists
who responded have used these
models — that’s higher than I would
have expected.
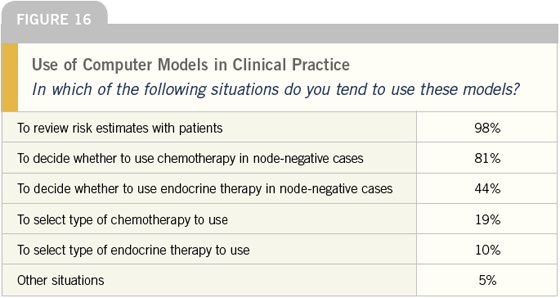
DR LOVE: You can see that in addition to
providing patients with risk estimates,
the most common use is treatment
decisions in node-negative cases. What
do you think that means?
DR O’SHAUGHNESSY: I believe it reflects
that clinicians are struggling with treatment
decisions, particularly in the
patients with receptor-positive, nodenegative
breast cancer. I use Ravdin’s
model to give patients a quantitative
estimate of their risk based on an
authoritative source rather than just
my opinion, particularly in these gray
areas.
DR LOVE: Bob, do you have any additional
thoughts on these numbers or the
use of these models?
DR CARLSON: I am really pleased that
the percentage of practitioners actually
using computer-based models is as high
as 60 percent. My expectation is that
the number is rapidly increasing and
it seems that people who have used
these models in practice use them
quite frequently. What made the use of
Adjuvant! widespread was distribution
— a diskette version is available at no
cost to medical oncologists across the
country.
I have found that it is difficult to
convince practitioners to try these
models; however, when they do, I believe
that they see the power of the numbers
and how the presentation of absolute
benefits to the patient can make
decision-making an easier and much
more objective process.
DR LOVE: I believe these models have
changed the culture of medical oncology
especially with regard to the way the
benefits of adjuvant therapy are being
presented in terms of absolute versus
relative risk.
DR CARLSON: I think you’re right.
Historically, most of us tried to present
both the relative and the absolute
benefits of therapy. But when it boils
down to decision-making, patients really
don’t care about relative benefits. They
want to know the absolute numbers.
Having a model like Adjuvant! allows us
to estimate absolute benefit much faster
and more reliably.
DR O’SHAUGHNESSY: I believe that’s
true, particularly in the low-risk
scenario. In the node-positive, higherrisk
situation, I still use the relative risk,
but when it comes down to one, two
or three points, I always use absolute.
While too many variables exist for these
models to be totally germane to one
patient, I believe as a general rule they
are very positive.
DR LOVE: Do you use the models
yourself?
DR CARLSON: I use these models for
every patient who comes to me for
a discussion of adjuvant therapy. For
the past two years I have printed out
the results and I usually give them to
the patient. I love the Adjuvant! model
because it helps me avoid biases. Many
factors influence how physicians think
about a specific patient — personality
type, type of relationship that is established,
referral source — these models
totally remove those from the equation.
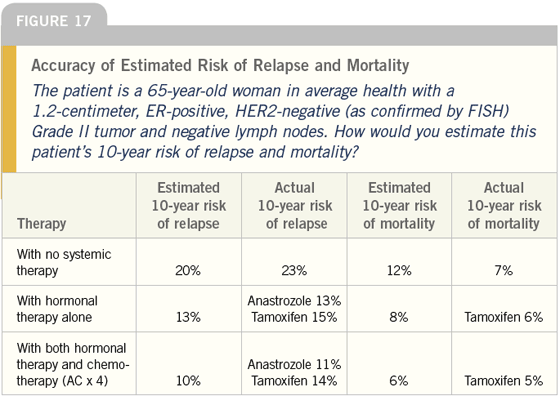
DR LOVE: We presented a case in this
survey (Figure 17) in which we asked
doctors how they would estimate the
patient’s 10-year risk of relapse and
mortality and then we calculated the
numbers using the Adjuvant! model. It’s
interesting how closely they match.
DR CARLSON: That surprises me too.
It makes me wonder if the use of these
models has resulted in more education
and now physicians are able to estimate
the absolute risk of relapse and of death
more accurately.
It would be fascinating to see this table
broken into two parts: one evaluating
the 60 percent of the practitioners who
said that they have used the computerbased
models and the other showing the
40 percent who have not used them. My
prediction is that we would see more
accuracy and consistency in the group
that uses these models than we do in the
group that does not.
DR LOVE: We actually did compare
those groups and, surprisingly, we did
not find many differences. Joyce, how
would you estimate the risk of relapse in
this patient?
DR O’SHAUGHNESSY: Without adjuvant
therapy, I believe this patient
has approximately a 12 percent risk.
Adjuvant therapy would cut that risk
in half; however, she would receive little
benefit from chemotherapy — possibly
zero benefit.
DR LOVE: The physicians who responded,
and Adjuvant!, gave higher estimates of
risk. Are they overestimating the risk?
DR O’SHAUGHNESSY: I fine tune risk
a bit more. The size difference in
Adjuvant! is between a 1.1-centimeter
tumor and a 1.9-centimeter tumor. I
estimate that a one-centimeter tumor
has a 10 percent risk of relapse and a
12-millimeter tumor has a 12 percent
risk of relapse.
DR LOVE: Regarding the question of
chemotherapy (Figures 18 and 19), I
am interested in your perspective on
what physicians are doing in practice
— especially the number that are using
dose-dense AC. Also what would be
your choices for chemotherapy in this
situation at varying ages?
DR CARLSON: I am surprised by how
many physicians are using AC alone,
especially for a very young woman with
what I would view as a substantial
risk of relapse. I would have thought
people would be more aggressive, either
adding a taxane or considering dosedense
adjuvant therapy. While all of
these therapies offer proportional risk
reductions, the addition of a taxane or
dose-dense therapy does increase that
risk reduction.
For me it would be a 50-50 split between
AC for four cycles every three weeks and
dose-dense AC followed by paclitaxel. I
have not used dose-dense AC without
paclitaxel in part because I believe if
the risk is great enough to warrant
the use of dose-dense therapy, then it
is presumably great enough to add the
taxane. I am cautious because we don’t
have any prospective randomized data
evaluating the utility of dose-dense AC
by itself.
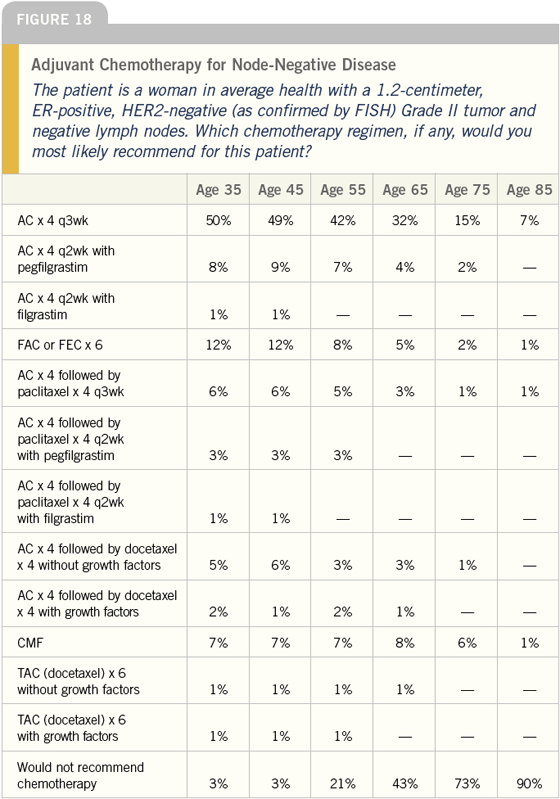
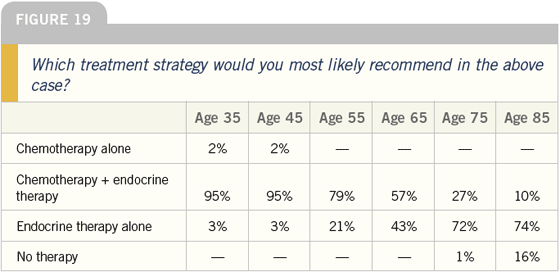
As the age of the patient increases,
obviously the magnitude of absolute
benefit decreases; therefore, I think as
women grow older, fewer are willing to
accept chemotherapy.
However, if you compare AC every three
weeks to AC followed by paclitaxel in a
dose-dense fashion, the magnitude of
benefit that you achieve by using AC is
at least matched by the addition of the
taxane and the dose-dense application
of it.
I think that most women who are willing
to accept AC for the absolute improvement
in benefit would also be willing to
accept the addition of a taxane in a dosedense
fashion.
In my practice a 65-year-old woman
would receive chemotherapy because
the benefits are relatively substantial. I
would offer her two alternatives — AC
or dose-dense AC followed by paclitaxel.
For an 85-year-old woman, however, I
think few of us would be anxious to give
her AC because of toxicity concerns.
DR LOVE: What about at age 75?
DR CARLSON: At age seventy-five it is a
tough discussion and is influenced by
comorbidities that may or may not exist,
the woman’s philosophy of life and how
much toxicity she would be willing to
tolerate for a very modest, but presumably
real, gain.
DR LOVE: Joyce, which chemotherapy
would you use in this clinical scenario?
DR O’SHAUGHNESSY: I generally don’t
use chemotherapy in postmenopausal
patients with ER-positive, node-negative
disease unless bad prognostic factors
exist, such as a tumor that is HER2-
positive, has a high proliferative fraction
or is Grade III.
DR LOVE: When you use chemotherapy
in a node-negative patient, which
regimen do you use?
DR O’SHAUGHNESSY: I generally use six
cycles of FAC. In the younger patient,
age 55 or younger, I discuss TAC with
the patient.
DR LOVE: Why TAC as opposed to AC/
docetaxel?
DR O’SHAUGHNESSY: When I use TAC
in that situation, I sometimes use only
four cycles. AC/docetaxel is six months
of therapy and if a patient is eligible
for that regimen, then I usually enroll
her in our clinical trial. I tend to use
AC/docetaxel in patients with highervolume
breast cancer, either T2 or nodepositive.
For patients age 65 and older, I
generally don’t use chemotherapy in this
scenario.
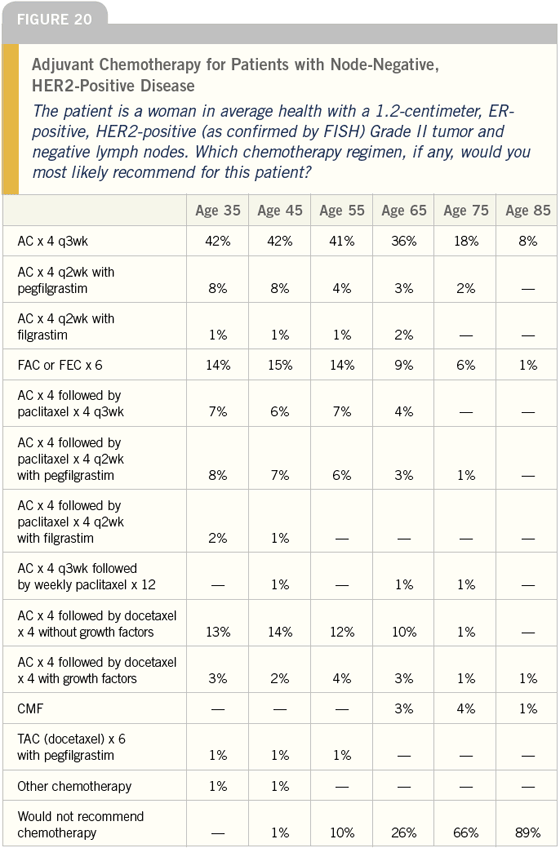
DR LOVE: How would you treat the same
patient if the tumor was HER2-positive
(Figure 20)?
DR O’SHAUGHNESSY: I would use an
anthracycline-based regimen for the
patient with HER2-positive breast
cancer.
DR CARLSON: It appears as though
physicians are much more likely to
use a taxane or dose-dense therapy for
patients with HER2-positive disease. I
think that is likely due to the perception
that women with HER2-overexpressed
breast cancer have a higher probability
of recurrence.
DR LOVE: Do you agree with that?
DR CARLSON: I think it is probably
true, but the available studies that have
examined the issue retrospectively are
somewhat contradictory. If we evaluate
this in one univaried analysis, HER2
is definitely prognostic. But after you
correct for lymph-node status, tumor
size, degree of differentiation and so on,
whether or not it remains independently
prognostic, I am not yet convinced.
DR LOVE: In your own practice, do
you tend to push your patients toward
taxanes a little bit more if their tumor is
HER2-positive?
DR CARLSON: I do, and I tell them
what I just told you. I also provide an
Adjuvant! estimate and explain that the
estimate does not incorporate the level
of HER2-overexpression. If their tumor
is HER2-overexpressed, the estimates
from Adjuvant! in terms of outcome
are high and, therefore, the estimates
for benefit from therapy are probably
slightly low.
DR LOVE: The next case (Figure 21) is
a woman in average health with a 1.2-
centimeter, ER-positive, HER2-negative
tumor and three positive nodes. This
is another situation in which we asked
physicians to estimate risk of relapse,
and here we see some disparity at least in
terms of the baseline risk of recurrence.
I would have guessed it would be a little
higher. Do you think a 34 percent risk
of relapse sounds right for this patient?
DR CARLSON: That is what I would tell
my patients because I quite literally use
Adjuvant! for every adjuvant patient I
see.
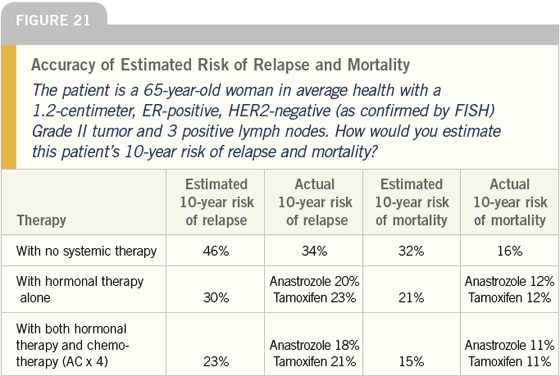
DR LOVE: It suggests that maybe a
prognosis of node-positive disease is not
as bad as we thought.
DR CARLSON: I think what it tells us is
that axillary lymph node status in isolation
is not as strongly predictive as we
would have expected.
In women with positive lymph nodes,
the degree of differentiation, hormonereceptor
status, size of the tumor and so
forth are also independently prognostic
and perhaps have a greater influence on
the ultimate prognosis than we previously
gave them credit for.
DR LOVE: That’s interesting. When you
use Adjuvant! within the node-positive
population do you notice significant
shifts based on grade and tumor size?
I have never played around with the
model in that respect.
DR CARLSON: Yes, you do see shifts and
it is not difficult to find patients who are
node-negative who have a risk of recurrence
that is greater than some of the
node-positive subsets.
I expect as these models are used more
and more over the next few years we will
see a culture shift and won’t be stratifying
patients in our brains or in our
practices based on nodal status as much
as we will base it on a risk estimate.
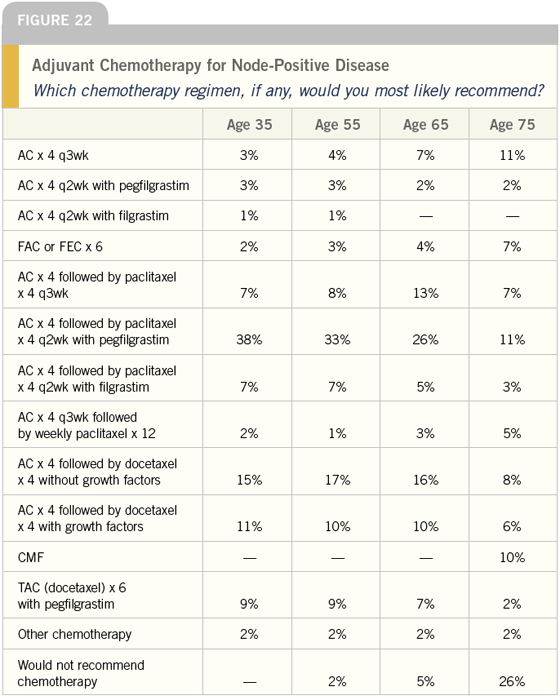
DR LOVE: Joyce, which chemotherapy
regimen would you use for a patient
like this with three positive nodes
(Figure 22)?
DR O’SHAUGHNESSY: My standard
treatment in this case would be AC
followed by docetaxel. I offer the same
regimen to patients ages 70 and over,
but I usually begin the docetaxel at 75
mg/m2 rather than 100 mg/m2. If they
do well, then I increase the docetaxel to
85 mg/m2.
One study I’m intrigued with is a trial
that Denise Yardley and Skip Burris
are planning, of TAC versus dose-dense
chemotherapy starting with docetaxel
at 100 mg/m2 every two weeks for four cycles followed by AC every two weeks
for four cycles. Filgrastim is used in this
trial.
DR LOVE: Wow! Has docetaxel every
two weeks been evaluated?
DR O’SHAUGHNESSY: Yes. George
Raptis published a paper in Anti-Cancer
Drugs evaluating preoperative docetaxel
100 mg/m2 every two weeks with a good
PCR rate demonstrating feasibility.
However, docetaxel 100 mg/m2 every
two weeks following AC is not feasible
because of skin toxicity, but you can
administer the docetaxel first. This
results in some skin toxicity, but it’s at
the tolerable level.
DR LOVE: What strikes me about the
use of chemotherapy for this type of
patient with node-positive disease is
the amount of dose-dense AC  T
being used and a reasonable amount of
docetaxel given in different ways. T
being used and a reasonable amount of
docetaxel given in different ways.
DR CARLSON: It is interesting to me how
quickly dose-dense AC  T has become
adopted and how widely it is used. I
view that as good news because it looks
like we’re doing a good job of translating
research findings into community
practice in a timely fashion. T has become
adopted and how widely it is used. I
view that as good news because it looks
like we’re doing a good job of translating
research findings into community
practice in a timely fashion.
DR LOVE: Joyce, what are your thoughts
about the dose-dense data?
DR O’SHAUGHNESSY: I’m the principal
investigator of the US Oncology trial
of AC followed by docetaxel versus AC
followed by docetaxel/capecitabine, so I
spend a lot of my day cheerleading for
that study. Therefore, I’m biased.
I’ve used dose-dense AC followed by
paclitaxel, but I tend to use it only
in patients whose disease is not high
enough risk to enroll in our clinical
trial. I have a patient who is a 40-yearold
nurse with a one-centimeter, Grade
III, Ki-67, 80 to 90 percent, ER-/PRand
HER2-negative tumor and negative
nodes.
While that’s not a “good” cancer, she
was not eligible for our trial because
the tumor was not staged as T1C. If
her tumor had been 1.1 centimeter,
she would have been eligible, but she
wasn’t eligible and I wasn’t going to treat
her with AC or FAC. She has a “bad”
cancer, so I gave her dose-dense AC
followed by paclitaxel.
I have another patient with multiple
myeloma in addition to breast cancer. I
plan to use hematopoetic growth factor
support, so I’m treating her with dosedense
AC followed by paclitaxel.
So, yes, I do use the dose-dense regimen
for selected patients.
DR LOVE: Bob, would you present AC
alone or CMF to a patient in this situation
as options?
DR CARLSON: If a woman who has
three positive lymph nodes has a risk
of recurrence that is substantial enough
in an absolute sense to warrant the use
of chemotherapy, then the addition of
the taxane or a dose-dense regimen or
both provides an advantage that is at
least equivalent to the addition of AC
or CMF.
If a woman is willing to accept AC for
four cycles for the absolute benefit she
will derive, then she is almost certainly
going to accept another four cycles of
treatment with a taxane.
DR LOVE: Do you ever use TAC off
protocol?
DR CARLSON: No, I don’t.
DR LOVE: It’s interesting that people
almost always use growth factors when
they use TAC. It seems that the message
has gotten through.
DR CARLSON: I think that is probably
one of the reasons why the TAC regimen
is not more widely used. People initially
didn’t use growth factors and had bad
experiences.
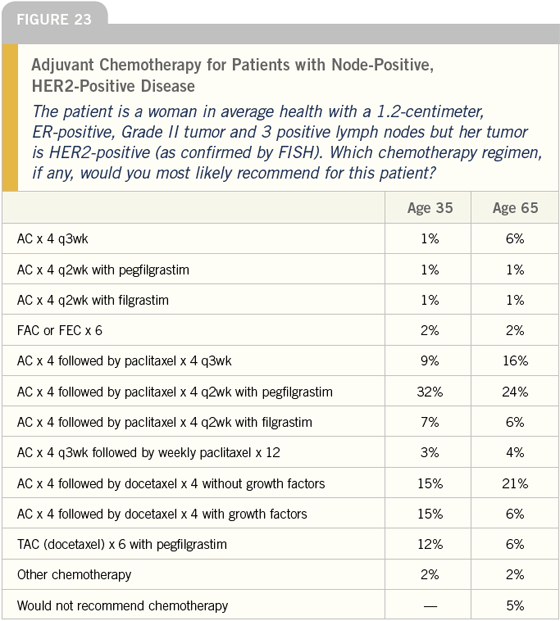
DR LOVE: Joyce, if this same patient were
HER2-positive, I assume that wouldn’t
change your treatment plan with chemotherapy.
Is that correct (Figure 23)?
DR O’SHAUGHNESSY: Correct.
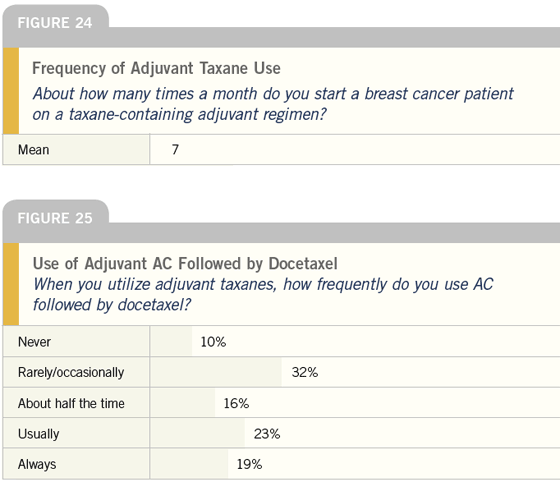
DR LOVE: We asked these clinicians how
many times a month they start a breast
cancer patient on a taxane-containing
adjuvant regimen, the response was
about twice a week (Figure 24). A significant
percentage of clinicians reported
they use AC followed by docetaxel for
adjuvant taxane therapy (Figure 25). Is
that your practice?
DR O’SHAUGHNESSY: In a nonprotocol
setting, my standard regimen is AC
followed by docetaxel.
DR LOVE: What has been your experience
with nanoparticle paclitaxel?
DR O’SHAUGHNESSY: I have treated
approximately seven patients with this
agent and I’ve found it’s extremely well
tolerated, particularly at the 100 mg/m2 dose. In the Phase II trial with 125
mg/m2, I had two patients who experienced
either significant fatigue or some
neuropathy with this higher dose.
I like the 100 mg/m2 dose because I see
very little myelosuppression or fatigue
and I can’t recall any patients experiencing
peripheral neuropathy.
DR LOVE: I assume you don’t premedicate
patients receiving nanoparticle
paclitaxel with the taxane.
DR O’SHAUGHNESSY: That is correct; I
don’t believe weekly dexamethasone is
good for patients — it tires them and
has a crash effect. Avoiding the premedication
may be one of the reasons why
we don’t see significant side effects with
nanoparticle paclitaxel.
DR LOVE: Would you use nanoparticle
paclitaxel in the adjuvant setting off
protocol?
DR O’SHAUGHNESSY: Without data, I
am not generally willing to substitute
nanoparticle paclitaxel for docetaxel
off protocol in the adjuvant setting. In
a middle-aged patient with numerous
comorbidities whom I can’t give TAC
because of the risk of febrile neutropenia
and other complications, I would consider every three-week nanoparticle
paclitaxel because it would be more
tolerable than docetaxel 100 mg/m2 and
I don’t feel dose-dense therapy is right
for every patient.
We are considering an adjuvant trial
comparing dose-dense AC followed by
dose-dense paclitaxel versus dose-dense
AC followed by dose-dense nanoparticle
paclitaxel.
I generally use docetaxel 100 mg/m2 in
the adjuvant setting. If I had a patient
who had been treated for early breast
cancer and had a recurrence with a
small, solitary lung metastasis that was
biopsy-positive — a pseudoadjuvant
setting — I would still use docetaxel.
I have two patients who have experienced
long-term complete responses
after six doses of docetaxel at 100
mg/m2; however, in patients who clearly
have metastatic disease, I am moving
toward using nanoparticle paclitaxel if
it is available.
DR LOVE: How do you think nanoparticle
paclitaxel compares with paclitaxel
and docetaxel in terms of efficacy?
DR O’SHAUGHNESSY: I believe nanoparticle
paclitaxel 260 mg/m2 is superior
to paclitaxel 175 mg/m2 in terms of
response rate and time to progression.
The data in the pivotal trial of
nanoparticle paclitaxel in anthracyclinepretreated
patients basically shows it to
be as efficacious as docetaxel in terms of
response rates.
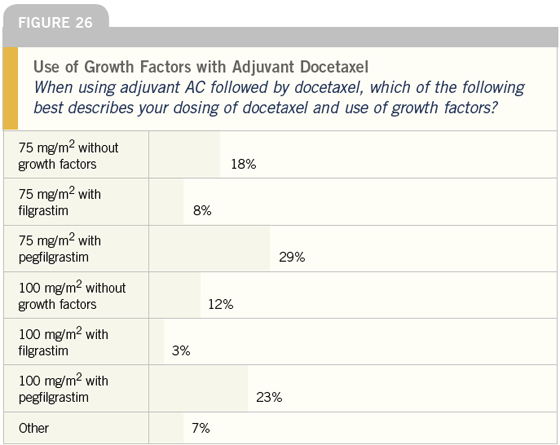
DR LOVE: When you use adjuvant
docetaxel, what dose do you use and
when do you use growth factors (Figure
26)?
DR O’SHAUGHNESSY: I generally use
100 mg/m2 of docetaxel. If I start any
lower, I begin with 85 mg/m2, particularly
in patients who are older, frail or
have multiple comorbid conditions.
I have started as low as 75 mg/m2,
although that is rare. I only use growth
factors if a patient requires it during AC
treatment and then I use pegfilgrastim
while continuing the AC. I never use
filgrastim.
DR LOVE: Can you comment on the
recent data regarding the incidence of
febrile neutropenia at 100 mg/m2 dose?
DR O’SHAUGHNESSY: In a large trial
of docetaxel 100 mg/m2 with over 700
patients, the 19 percent rate of febrile
neutropenia was reduced to one percent
with pegfilgrastim. I believe it included
patients with metastatic disease. We use
AC followed by docetaxel in the adjuvant
setting and less than 10 percent, maybe
eight or nine percent, of our patients
experience febrile neutropenia.
DR LOVE: Why is febrile neutropenia
more common in metastatic disease?
DR O’SHAUGHNESSY: A number of
possibilities exist. With liver metastases,
even if the patient’s liver function studies
are normal, we still wonder about their
metabolism. Also, if the patient has
received other chemotherapeutic agents,
the integrity of the mucous membranes
in the gut may not be 100 percent.
Also, we know that as a patient’s performance
status declines, drug metabolism
and excretion may not be as robust and
if a patient is symptomatic from their
breast cancer, their nutritional status
may not be optimal.
DR LOVE: If you could reduce the rate of
febrile neutropenia from eight or nine
percent to one percent in the adjuvant
setting, why not use growth factors?
DR O’SHAUGHNESSY: Whenever we
treat a patient, we want to have an
evidence-based reason to do so and we
have little data on these agents in the
adjuvant setting.
We have a good deal of experience in
using hematopoietic growth factors in
metastatic patients, but, unfortunately,
their life spans are too short to determine
the long-term effects of these
agents.
In the adjuvant setting, most of these
patients will be cured, but they’re
receiving alkylators, anthracyclines and
many receive radiation therapy and the
long-term risk of leukemia is unknown.
Filgrastim was used in the NSABP
studies B-22 and B-25 and elevated
risks of leukemia occurred, but they
were also using high doses of cyclophosphamide.
In addition, it doesn’t make
economic sense to use an agent in all
patients that is not needed 90 percent
of the time.
Select publications
|

