| |
Chemotherapy for Metastatic Disease |
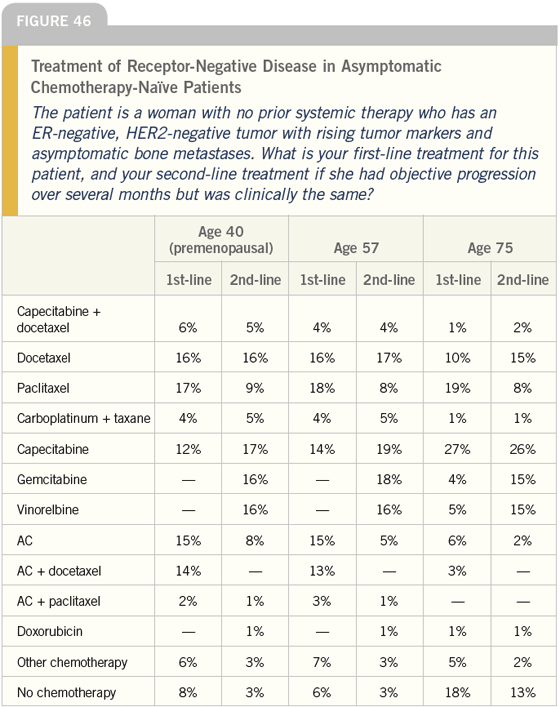
DR LOVE: Bob, I’ll go through a few of these metastatic disease scenarios with you. For a patient with ER-negative, HER2-negative metastatic disease with asymptomatic bone metastases who has never received systemic therapy, which regimens would you typically use firstand second-line (Figure 46)?
DR CARLSON: I think I am consistent with the responses to the survey, in that I am remarkably inconsistent and do not follow a single regimen. Little evidence exists to suggest that any one chemotherapy regimen provides a meaningful advantage to the woman in terms of response rates, duration of response, survival and so on, relative to other combinations or single agents.
I tend to discuss what she expects from her treatment, how much toxicity she is willing to tolerate and when she would be willing to do so; however, in an asymptomatic woman, I try to minimize toxicity. Why should I make a woman sick when she feels well?
In this situation, I often start with an agent such as capecitabine, and that would be independent of age. However, I can’t be critical of any of the choices that have been made.
DR LOVE: What tends to be your secondline therapy?
DR CARLSON: A taxane.
DR LOVE: Which one?
DR CARLSON: Again, I discuss the type of experience she wants and try to gauge how “toxic” she views coming to my office. When I use paclitaxel, I tend to use it weekly and when I use docetaxel, I tend to use it every three weeks. In general, I think docetaxel is a slightly more toxic agent than paclitaxel, so, to some extent, it becomes an issue of toxicity of treatment versus the number of visits the patient is willing make.
DR LOVE: How much, if any, is your decision-making influenced by age?
DR CARLSON: Age influences me primarily in the same way that it does the survey respondents. The older a woman is, the more likely I am to use a single agent because single agents alone tend to be less toxic.
DR LOVE: Looks like there’s a shift toward capecitabine with increasing age. Is that your approach?
DR CARLSON: Sure, because I will shift to a single agent due to toxicity concerns. Capecitabine tends to have manageable toxicity. Although, I do think it is a mistake to look at capecitabine as a nontoxic chemotherapy.
DR LOVE: Approximately one third of these docs picked combination therapy as their first-line choice for an asymptomatic 40-year-old woman. Do you think that is a reasonable recommendation?
DR CARLSON: I think it’s reasonable in that there is no data to show that it is a disadvantage to the woman with regard to the most important endpoint — survival. In fact, limited data from imperfect studies suggest combination chemotherapy may provide a slight survival advantage. I guess I’m surprised that people would step up to the plate with aggressive combination regimens in an asymptomatic woman with boneonly metastatic disease, especially with bone being such a favorable site for metastases.
DR LOVE: If nanoparticle albuminbound paclitaxel were available, would you use it as first-line therapy in the metastatic setting?
DR CARLSON: Yes. Data indicate that nanoparticle paclitaxel is at least as efficacious as paclitaxel, and perhaps slightly more so. The toxicity experience with this new agent is also convincing, especially in terms of hypersensitivity reactions.
I think it would be beneficial if we could diminish the need for relatively expensive antihypersensitivity medication. As far as neuropathy, I think we are going to need a lot more data to really understand whether nanoparticle paclitaxel has either less, or more, rapidly reversed neuropathy than paclitaxel.
DR LOVE: Any guess on efficacy or side effects with nanoparticle paclitaxel compared to docetaxel?
DR CARLSON: My expectation is that it will be similar to the data with paclitaxel. Docetaxel has frequent hypersensitivity reactions, just like paclitaxel, and being able to avoid dexamethasone, diphenhydramine and so forth, is a very reasonable goal, if we can accomplish it.
DR LOVE: You talked about the cost savings involved in avoiding hypersensitivity medications, what about the quality-of-life impact of avoiding these side effects? How much of a benefit would that be?
DR CARLSON: I think the side effects of those medications are an issue. It doesn’t take many cases of aseptic necrosis to make avoiding dexamethasone a really good goal. Likewise the sedation that goes along with diphenhydramine is also a potential problem we would like to eliminate.
DR LOVE: If it were available, are there any circumstances that would lead you to use adjuvant nanoparticle paclitaxel in a nonprotocol situation? If not, would it be your first-line taxane in the metastatic setting?
DR CARLSON: I would not use it in the adjuvant setting; however, I think it would be a reasonable first-line choice for metastatic disease. Whether or not I would use it in the six months after it became approved, rather than paclitaxel and docetaxel, with which we have an incredible wealth of experience, I guess I’d have to see.
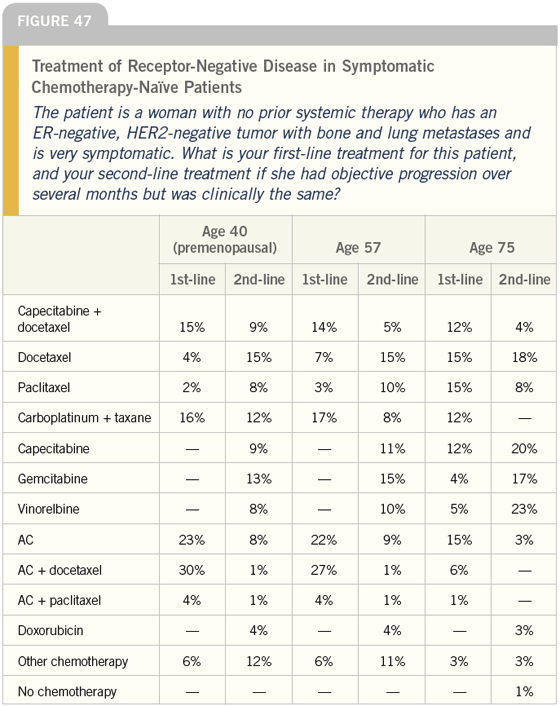
DR LOVE: Let’s go to the next case. How would you approach a symptomatic patient like this one and what would your first- and second-line therapies be (Figure 47)?
DR CARLSON: I would certainly be much more inclined to use combination chemotherapy because the probability of response goes up, and it sounds like this patient needs a response. I would personally use AC, CAF or FAC, one of those types of regimens as first-line therapy.
DR LOVE: How about in a 75-year-old woman?
DR CARLSON: A 75-year-old woman obviously presents a much more difficult situation. Again, you need to consider how sick you are going to make her compared to how sick she is feeling.
In that situation I would probably go with AC.
DR LOVE: Here (Figure 48) we have a situation similar to one we talked about before — an asymptomatic patient with bone metastases. However, this patient received adjuvant AC 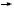 paclitaxel two years ago. In general, what would you be inclined to do with this patient? paclitaxel two years ago. In general, what would you be inclined to do with this patient?
DR CARLSON: A spectrum of options exist. Given her prior exposure, I think it makes sense that respondents tend to use fewer anthracycline-based regimens. I’m surprised by the frequency of use of the taxanes.
Data suggest that complete cross resistance does not occur between paclitaxel and docetaxel, but some cross resistance does occur. In general, I would use capecitabine as my first-line therapy.
DR LOVE: It’s interesting to see so much variation in all of these cases. You can almost imagine a woman going to five different doctors and receiving five different therapies.
DR CARLSON: Yes, but that is not surprising. When you look at the endpoints that are most important — survival and progression-free survival — little data show that these regimens differ from one another. What shapes these decisions are the complex motivations of patients and physicians.
DR LOVE: One of the things I noticed was a big shift to capecitabine/docetaxel when we asked about the symptomatic patient previously treated with adjuvant AC 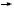 paclitaxel. paclitaxel.
DR CARLSON: I find capecitabine to be an active agent that is relatively easy to administer, and I would consider using it as a single agent. If you are going to add a taxane, which is a reasonable thing to do, I think docetaxel makes more sense than paclitaxel.
I am surprised by the frequent use of carboplatin, in combination with a taxane. These types of regimens are modestly toxic and very expensive. If you are going to use combination therapy and accept a fair amount of toxicity, then the carboplatin/paclitaxel in preference to capecitabine/docetaxel doesn’t make a lot of sense to me.
I haven’t seen patterns of care data like this, other than some relatively modest data from National Comprehensive Cancer Network (NCCN) centers. I think this type of information is interesting and can help highlight where the educational opportunities are and what the future research questions should be.
A great example is the issue we just talked about — should we try to better define the optimal first-line regimens for metastatic disease? I think that is a good question, and your data tells us that this is a problem.
DR LOVE: This type of survey may also help us determine what data is needed to help docs make better decisions. Trials that extend survival and progressionfree survival have always been emphasized, but quality-of-life issues exist for which I’m not sure we have much data.
DR CARLSON: We don’t, and that is a very important point. It is actually my major consideration in trying to help a woman with metastatic breast cancer select one chemotherapy versus another.
I tell patients up front that they are going to receive a list of drugs over the course of their treatment, and that the major issue we will deal with is not which drugs, but the sequence in which they will be used. For the most part, the sequence is based more on toxicity considerations than on efficacy.
DR LOVE: The next case (Figure 49) involves a symptomatic patient with metastatic disease who has previously been treated with AC 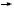 paclitaxel in the adjuvant setting. The number of physicians treating with chemotherapy combinations (capecitabine/docetaxel and a platinum/taxane combination) is much greater in a patient like this. paclitaxel in the adjuvant setting. The number of physicians treating with chemotherapy combinations (capecitabine/docetaxel and a platinum/taxane combination) is much greater in a patient like this.
Joyce, could you comment on the recent data from the randomized study of paclitaxel with or without gemcitabine in women with metastatic breast cancer?
DR O’SHAUGHNESSY: This trial demonstrated a survival advantage for adding gemcitabine. It was an interim survival analysis requested by the FDA prior to the registration of gemcitabine. This positive survival advantage is important because it gives us another agent that can impact the natural history of metastatic disease, and the current list of efficacious agents in this setting is short.
It’s now important to study gemcitabine in the adjuvant setting, which is being done in the TANGO trial in the United Kingdom and NSABP-B-38.
In the metastatic setting, we still need to know whether we should treat patients with this combination up front, as was done in this trial, or further down the line. I believe either option is appropriate and selection depends on the tumor burden and symptomatology.
DR LOVE: How do you feel about this combination in the nonprotocol setting as opposed to capecitabine/docetaxel or capecitabine/paclitaxel as recently reported by Gradishar?
DR O’SHAUGHNESSY: I don’t generally use paclitaxel every three weeks as Bill Gradishar did in his Phase II trial. We are conducting a Phase II trial of weekly paclitaxel 80 mg/m2 on days one and eight, with day 15 off in a 21-day cycle, along with capecitabine 1,650 mg/m2 daily, 14 days on and seven days off.
We have completed the front-line cohort in patients who are taxane-naïve, and Joanne Blum will present those data in a poster at the 2004 San Antonio Breast Cancer Symposium. It’s a wonderful combination — active and safe. As for the cohort of patients who are taxane pretreated, we have 15 more patients to accrue.
In my practice, whether I use capecitabine in combination with docetaxel, or paclitaxel alone, depends on which taxane the patient has already received. Historically, I’ve been using capecitabine/docetaxel in patients with a heavier tumor burden or patients who are very concerned about their metastatic disease and want the best chance for a durable remission.
For patients with indolent disease, I generally use single-agent capecitabine followed by a taxane.
The gemcitabine/paclitaxel data is relatively new and I’m still trying to determine the best use of that regimen. Until now, I’ve been saving gemcitabine to combine with carboplatin.
That’s an active combination and I’ve been pleased with the responses, particularly in drug-resistant patients; however, I’m finding cumulative thrombocytopenia to be a problem after three to five treatments.
This toxicity requires prolonging the interval between cycles and then the patients lose their response.
While gemcitabine and carboplatin are both important drugs, I’m rethinking which agents to combine them with and I find I’m swinging back to combining gemcitabine with a taxane or vinorelbine, as I did a year and a half or two years ago.
Gemcitabine partners well with other agents because it doesn’t add substantially to toxicity. I’ve used it either with docetaxel, paclitaxel or vinorelbine, generally as second- or third-line therapy for metastatic disease. These are welltolerated active combinations.
DR LOVE: What about the combination of gemcitabine with either capecitabine or nanoparticle paclitaxel?
DR O’SHAUGHNESSY: The combination of capecitabine with paclitaxel for two out of three weeks is active and well tolerated. I believe gemcitabine or carboplatin combined with nanoparticle paclitaxel will provide nice palliation for patients. The combination of nanoparticle paclitaxel/carboplatin is also being studied with trastuzumab.
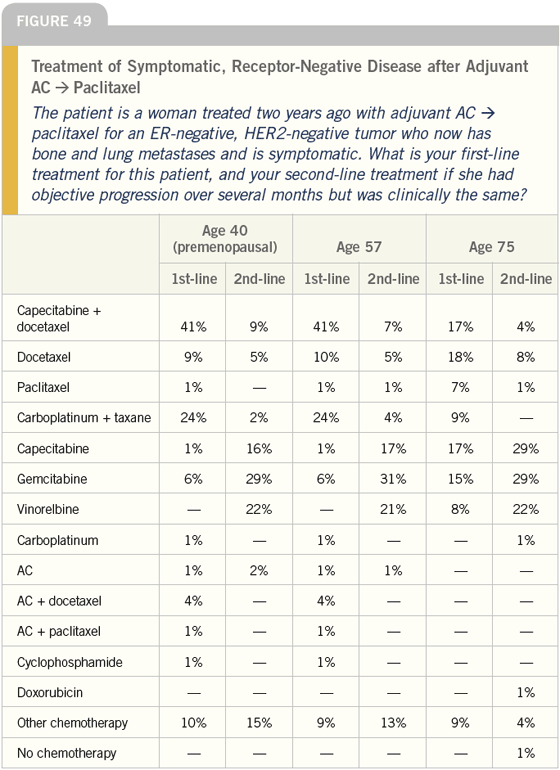
DR LOVE: Joyce, let’s look at more data on the use of docetaxel in the metastatic setting. How do you dose this agent for patients with metastatic disease (Figure 50)?
DR O’SHAUGHNESSY: It depends on the setting. With chronic use, I give 75 to 85 mg/m2 every three weeks to avoid the neurotoxicity we see with weekly use; however, if I’m only going to give patients four to six cycles, I use 100 mg/m2.
DR LOVE: And would you use prophylactic growth factors with docetaxel in the metastatic setting (Figure 51)?
DR O’SHAUGHNESSY: Based on the data showing a 19 percent rate of febrile neutropenia in metastatic disease, I would definitely use prophylactic growth factors with docetaxel 100 mg/m2. On the other hand, I find 75 mg/m2 is well tolerated; I’ve not seen a significant incidence of myelosuppression with that dose.
DR LOVE: Where do you see nanoparticle paclitaxel being utilized in the treatment of breast cancer over the next couple of years?
DR O’SHAUGHNESSY: I believe physicians will use nanoparticle paclitaxel for palliation in the metastatic setting in patients whom they want to experience as few side effects as possible. I expect it will be used weekly at 100 mg/m2 for three weeks, followed by one week off, as in Joanne Blum’s study.
I believe it will be a popular choice to avoid the on-again, off-again fatigue of weekly dexamethasone and for patients with any kind of peripheral neuropathy. It’s so well tolerated overall that I expect it will be favored over weekly paclitaxel.
DR LOVE: Do you believe physicians will prefer it over docetaxel?
DR O’SHAUGHNESSY: I’m not certain how it will compare with docetaxel. Often a patient with metastatic breast cancer has received both taxanes — one in the adjuvant setting and the other in metastatic disease.
If a patient has received adjuvant paclitaxel, depending on how long ago she received it, I believe weekly nanoparticle paclitaxel will be seriously considered over docetaxel because of its tolerability.
Select publications
|

