|
Click here to see image
Colorectal Cancer Update 2004 (6) |
DR HALLER: Since the 1991 consensus
conference, the American model for the
management of rectal cancer had generally
consisted of surgery followed by
postoperative chemoradiation therapy
for patients with Stage II or Stage III
disease, but I now believe that preoperative
chemoradiation therapy is the gold
standard for patients with rectal cancer.
Currently, both adjuvant and
neoadjuvant chemoradiation therapy are
acceptable options, but based on the
German Rectal Cancer trial comparing
preoperative and postoperative chemoradiation
therapy, more people will be
switching to the preoperative model. In
the United States, preoperative or postoperative
radiation therapy alone would
be an unacceptable option.
We are mostly using neoadjuvant
chemoradiation therapy, so I believe
a standard regimen would consist of
infusional 5-FU and radiation therapy.
According to Joel Tepper’s presentation
of the Intergroup-0114 trial results, the
bolus 5-FU regimens have more toxicity
and equal efficacy compared to the infusional
regimen; however, patients who
are confounded by infusional therapy
might choose one of the bolus regimens.
In patients who do not want infusional
therapy, the cumulative data for
capecitabine suggest that it could be
substituted. I’m not willing to simply say
capecitabine can be substituted in every
patient, but I believe it’s an option.
Colorectal Cancer Update 2005 (5) |
DR LOVE: What is your general approach
for neoadjuvant chemotherapy for
patients with rectal cancer?
DR GROTHEY: The Mayo Clinic is a
conservative institution, and we are using
continuous-infusion 5-FU in this situation,
but I think the data are compelling
that capecitabine can be used as a
substitute. Outside of clinical trials, we
shouldn’t be afraid to use capecitabine.
Having said that, this is currently being
investigated in NSABP-R-04, which
compares radiation therapy with either
capecitabine or infusional 5-FU.
A second randomization will evaluate
the addition of oxaliplatin. The
future involves increasing the efficacy of
neoadjuvant chemotherapy because in
the end, patients eventually succumb to
distant metastases.
Adding more effective chemotherapy
up front in combination with radiation
therapy will allow us to maintain
systemically active chemotherapy, which
might attack micrometastases as early
as possible.
I’m sure it will enhance the pathologic
complete response rate following chemoradiation
therapy, which is a predictor
for overall survival. Hence, we’ll have
local control improvement, and with the
use of combination chemotherapy early
on, we might have an impact on distant
metastases.
DR LOVE: Can you describe a rectal
cancer case where you would not recommend
neoadjuvant therapy?
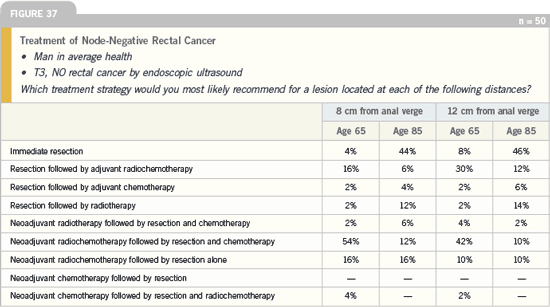
DR GROTHEY: In a situation where the
lesion is high — 12 centimeters from the
anal verge — I would recommend resection
followed by chemotherapy. However, the majority of respondents selected
neoadjuvant radiochemotherapy. The
decision not to utilize neoadjuvant therapy
is determined by the location of the
lesion. The higher the tumor is located,
the less important radiation becomes.
Click here to see image
In discussing neoadjuvant strategies,
the key issue is the radiation component,
and if we want to extrapolate, we have
some data from the Dutch trial looking
at the location of the tumor relative
to the risk of recurrence. For a patient
who has a tumor located 12 centimeters
from the anal verge, recurrence rates —
even without radiation — are less than
five percent.
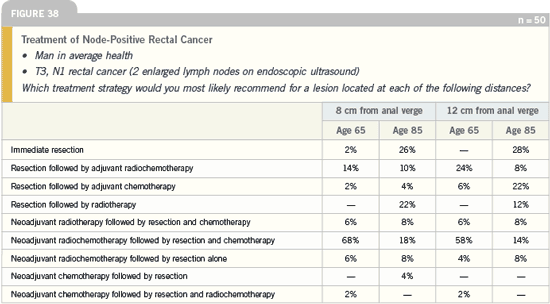
DR LOVE: In terms of the general recommendation
for neoadjuvant therapy in
patients with rectal cancer, most physicians
use intravenous 5-FU as opposed
to capecitabine. What is your opinion
on that?
DR GROTHEY: This is clearly because
we don’t have any Phase III neoadjuvant
data with capecitabine in rectal cancer
available yet. I was actually surprised that
for older-age patients, the recommendation
for capecitabine increased to almost
one fourth of patients. This is interesting,
and it shows potential for this drug
because apparently it’s perceived as more
tolerable for elderly patients. If we are
able to document that capecitabine is
equally effective — which is a matter
of ongoing trials — then it could be a
nice replacement for intravenous 5-FU.
I think in the future we will see more
usage of CAPOX.
The primary reason we use capecitabine
is patient convenience. I believe
that 5-FU and capecitabine are interchangeable
in terms of efficacy. In dosing
capecitabine, I tend to use capecitabine
twice a day Monday through Friday during
radiation as a radiosensitizer. We tell
patients not to use multivitamins while
they are taking capecitabine.
DR LOVE: How about CAPOX in the
neoadjuvant setting?
DR GROTHEY: To be honest, I thought
it would have been used more. We do
have published data on that, including
results published in the Journal of Clinical
Oncology over two years ago. So, you
could use it with the idea that oxaliplatin
adds significant efficacy in terms of
the systemic recurrence. However, based
on this survey, it does not appear that
CAPOX is utilized in the neoadjuvant
setting for rectal cancer.
Colorectal Cancer Update 2005 (4) |
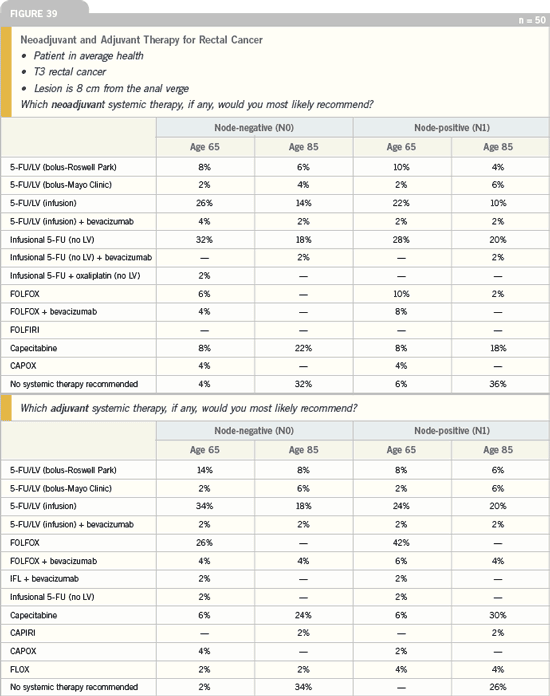
DR LOVE: What do you think about
NSABP-R-04, evaluating preoperative
radiotherapy with capecitabine versus
5-FU and the second randomization to
add oxaliplatin?
DR PHILIP: I have mixed thoughts
with respect to the first randomization
in NSABP-R-04 of infusional
5-FU or capecitabine because I already
use capecitabine with radiation therapy.
We started using this at our institution
several years ago when there weren’t any
protocols available. We reviewed and
published our experience confirming the
safety of this approach; therefore, we
have been using capecitabine routinely in
these patients in the clinical setting.
I am interested in the second randomization
in the trial using oxaliplatin.
I have started using that in combination
with radiation therapy in some,
but not all, patients. For example, I have
used oxaliplatin in healthier patients with a better performance status and in
patients with whom I have special concerns
about not being able to preserve
the sphincter, where I want to obtain a
maximum pathologic response.
Click here to see image
Click here to see image
Colorectal Cancer Update 2005 (5) |
DR CHRISTOPHER CRANE: The NSABP
designed R-04 to compare capecitabine
with venous infusional fluorouracil in
patients receiving preoperative radiotherapy
for locally advanced rectal cancer, but
I don’t believe such a trial is necessary.
The study design has now been changed
to incorporate oxaliplatin, which I
believe is our only opportunity to understand
whether that drug will benefit such
patients. The final design is a two-by-two
randomization of infusional 5-FU versus
capecitabine with a second randomization
to oxaliplatin or not.
I believe everyone will agree that
the amended design is better. If I had
to guess what this trial would show,
my guess would be that capecitabine
will be equally effective but less toxic
than infusional 5-FU and that oxaliplatin
will improve response but not longterm
outcome.
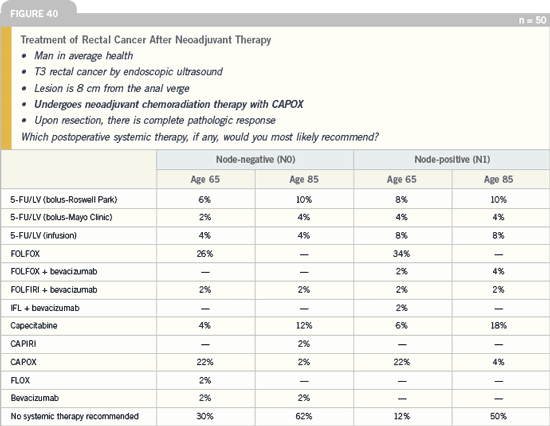
DR LOVE: What about the role of
neoadjuvant bevacizumab in the treatment
of rectal cancer?
DR CRANE: Bevacizumab has been proven
in many disease sites to improve
the effects of chemotherapy. Approximately
three years ago, before it was
approved with radiation therapy, we had
the opportunity to investigate this agent.
We conducted a Phase I trial of 50
patients with pancreatic cancer (ID02-146) who received capecitabine, radiation
therapy and bevacizumab, and the
results were very exciting. In the patients
who received five mg/kg of bevacizumab
every two weeks, which was the final
recommended dose, we saw a 50 percent
partial response rate. Six of the 12
patients had their tumors shrink by 50
percent, which is a “high bar” endpoint
for pancreatic cancer.
The regimen was well tolerated, and
the RTOG is now conducting a Phase
II study with bevacizumab, capecitabine
and radiation therapy in patients with
locally advanced pancreatic cancer that
cannot be surgically excised (RTOG-0411).
Click here to see image
At MD Anderson, we currently have a neoadjuvant Phase II study with
the same regimen in patients presenting
with locally advanced rectal cancer.
Investigators at Mass General published
a Phase I trial in Nature Medicine and
presented it at ASCO in 2004. In this
trial, patients with primary rectal cancer
received neoadjuvant bevacizumab,
5-FU and radiotherapy.
It was initially reported that five out
of six patients had either microscopic
residual or complete pathologic responses
to the preoperative regimen, and I know
from personal communication that these
results are holding up, and now 11 out
of 12 patients have had this response. In
addition, no surgical catastrophes have
been encountered following this regimen
as long as six weeks elapse before the
patient undergoes surgery.
These data open a lot of doors for the
future of these patients and chemoradiation
in general. In clinical trials, we will
be evaluating bevacizumab’s ability to
enhance the effect of radiation therapy.
One of our focuses at MD Anderson
is organ preservation, and with bevacizumab,
instead of removing radiation
therapy from the neoadjuvant treatment
equation, this agent, when used with
radiation therapy, may lessen how radical
a surgery needs to be. I want to stress that
this is investigational, but the responses
are better, and I believe they will also
translate into better local control.
Colorectal Cancer Update 2005 (6) |
DR VENOOK: Bevacizumab obviously
has great potential in the rectal cancer
setting. Chris Willett’s paper in Nature
Medicine was a very clever and interesting
development. These were patients
with primary or locally advanced rectal
cancer who received a single dose of
bevacizumab and, in 12 days, were reevaluated
with imaging and biopsy and
then received 5-FU/radiotherapy. Blood
flow, blood volume and tumor vasculature
all were impacted by a single dose
of bevacizumab to these tumors that
were in situ. So certainly, there’s biological
activity of bevacizumab alone, and I
think this really needs to be looked at in
neoadjuvant studies.
DR HOFF: We try to put these patients
on protocol as much as possible. Right
now, we have a preoperative Phase II
protocol with capecitabine, bevacizumab
and radiation therapy. If patients cannot
participate in this study, we usually use
preoperative capecitabine with radiation
therapy.
Colorectal Cancer Update 2004 (4) |
DR LOVE: What are your thoughts
regarding bolus 5-FU, infusional 5-FU
and capecitabine for the adjuvant treatment
of rectal cancer?
DR WOLFF: In the adjuvant setting,
when infusional 5-FU rather than bolus
5-FU is combined with radiation therapy,
disease-free and overall survival are
improved. Infusional 5-FU is a better
radiosensitizing agent. The advantage of
infusional 5-FU with radiation therapy
is probably more of a systemic than a
local control benefit.
An Intergroup trial published in The New England Journal of Medicine demonstrated
a trend toward better local
control using infusional 5-FU compared
to bolus 5-FU. That study is proof of the principle that infusional 5-FU is a superior
treatment modality when combined
with radiation therapy.
Capecitabine is an interesting alternative
to infusional 5-FU for several
reasons. With infusional 5-FU, catheterrelated
problems can develop, such as
thrombosis and infection. Additionally,
patients are required to carry an ambulatory
pump. When the pump is on for a
couple of weeks it’s no big deal, but generally
by the fifth week of radiation therapy,
patients are tired of it. Capecitabine
is a nicer route of administration.
Additionally, capecitabine is a prodrug,
and it has to be converted to
5-FU at the intracellular level. One of
the enzymes responsible for that conversion
is thymidine phosphorylase (TP),
which is expressed in higher concentrations
in the rectal mucosa.
At the biological level, that may mean
that the rectum, the rectal mucosa and
the tumor cells have a higher intracellular
concentration of 5-FU, leading
to both an active cytotoxic benefit
and more radiosensitization. We have
therefore been interested in evaluating
capecitabine as a radiosensitizer compared
to infusional 5-FU.
In the future, we will combine
capecitabine with bevacizumab. That
trial is not yet open, but we will pursue
not only conventional cytotoxic agents
with radiation but also utilize biologic
agents such as bevacizumab for this
group of patients.
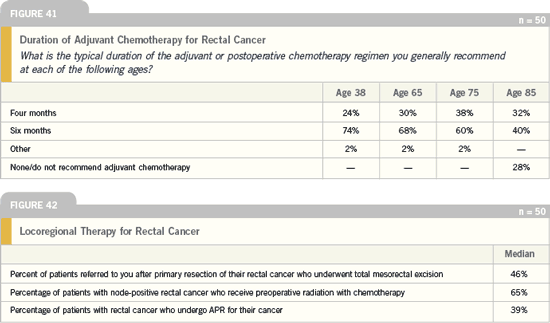
DR LOVE: What is your opinion of the
survey findings related to locoregional
therapy for rectal cancer?
DR GROTHEY: The number of patients
who have undergone total mesorectal
resection — 50 percent — seems to
be quite low. Interestingly, the number
of patients who have undergone APR
seems a little bit high — it should be
under 20 percent.
Of course, this could be due in part to
the definition of what qualifies as rectal
cancer. Is the 12-centimeter tumor still
rectal cancer, or is it sigmoid cancer?
This should be factored into the equation;
when the oncologists answered the
question, were they only thinking of low
rectal cancers? We define rectal cancer
as a lesion located between the anal verge
and up to 12 centimeters from the anal
verge. Our goal is to reduce the need for
APR to less than 20 percent.
Select publications
|

