| |
Editor’s Note: Consensus and controversy |
After many years of conducting
patterns of care surveys in breast
cancer, we are very pleased to
present our first initiative outside of that
tumor type. This monograph delivers
the results of a national telephone survey
of 100 randomly selected US-based
medical oncologists, who detailed their
likely nonprotocol treatment recommendations
for a variety of colorectal cancer
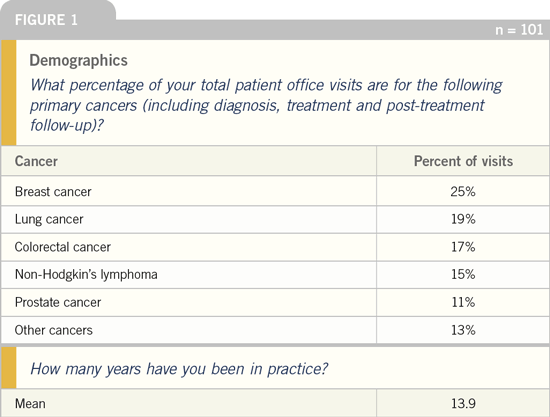
case scenarios. Colorectal cancer represents
one in six office visits for these
physicians, who have been in practice an
average of about 14 years (Figure 1). As
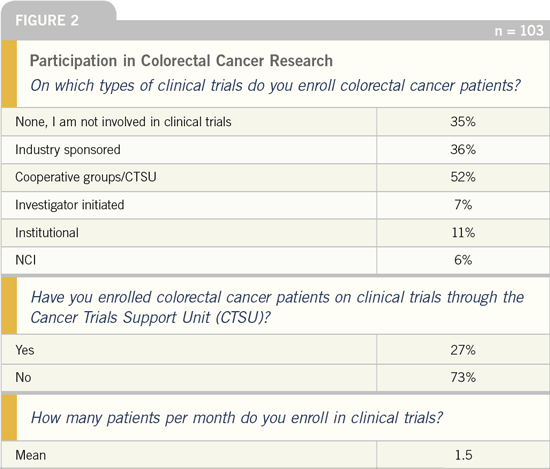
in prior similar surveys, about two thirds
of these clinicians actively participate in
clinical trials, with both industry and the
government (Figure 2).
One of the most striking features of
the survey data is the mixture of consensus
and heterogeneity in the responses of
these docs.
It is interesting to consider that in
some of the survey scenarios, if a patient
were to seek a second, third, fourth
and even fifth opinion, it is possible or
even likely that multiple, very different
treatment plans would be suggested,
with great variation in the personal and
financial costs and perhaps in the antitumor
efficacy.
How about 99 second opinions?
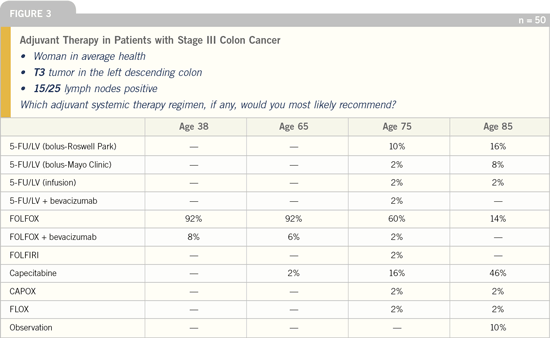
Figures 3 and 4 provide two examples of
what we found in our current colorectal
cancer survey. Of note are a number of
situations in which a current consensus is
apparent, but in other situations, there is
considerable heterogeneity of responses.
Figure 3 is an example of a situation
in which a consensus exists, specifically
with regard to adjuvant systemic therapy
for younger patients with Stage III
disease. Clearly the FOLFOX message
has been transmitted and received, yet
one could wonder why the FOLFOX
answer to this question is not 100 percent.
However, in more than 20 years of
conducting these surveys in breast cancer,
we have consistently observed that even
in situations where the clinical research
community is unified in their treatment
approach, there is always a small fraction
of oncologists — usually at least 10 percent
— taking another path. Part of this
variability could be a function of inaccuracies
in such informal surveys, but in my
opinion these outliers are for real.
It would be interesting to evaluate
whether this small group is familiar with
current clinical research data and disagrees
with the perspectives of colleagues could benefit by more effective continuing
education. I believe the answer is a
combination of these two factors.
Figure 3 also demonstrates another
consistent finding in our surveys: The
diversity of treatment recommendations
tends to increase with age and is particularly
divergent in octogenarians. Note
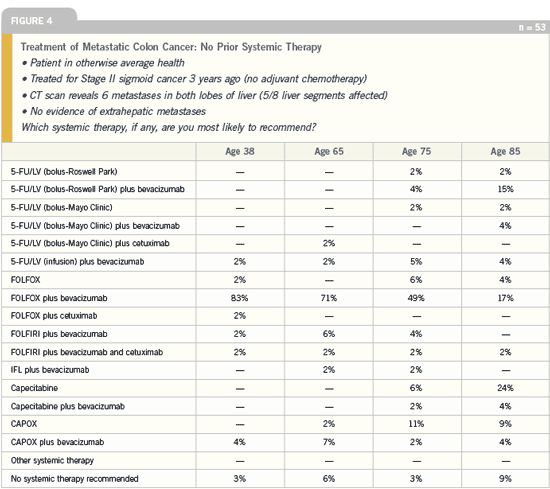
that in Figure 4, which focuses on metastatic
disease, in the scenario of the 85-year-old patient with metastatic disease,
more than a dozen treatment approaches
are utilized, ranging from no systemic
therapy to combination chemotherapy
and biologic treatment.
In our breast cancer Patterns of Care
series, we recently conducted two simultaneous
studies involving both oncologists
in practice and clinical investigators
specializing in breast cancer. As one
might expect, there was a greater degree
of consensus among the investigators
than among the community practitioners.
It would be interesting to launch a
parallel effort in colorectal cancer, and I
suspect there would be similar findings.
What does this all mean and how,
if at all, is it relevant to efforts in continuing
oncology education and patient
counseling?
From a CME perspective, we think
that the data reinforce the need for casebased
education, such as our Meet The
Professors audio series. The roundtable
format that juxtaposes clinical investigators
and community-based oncologists
is ideal for discussing the application
of clinical research information to daily
treatment decisions. In preparing for
these recordings, I conduct one-on-one
teleconferences with each community
doc, in which we review cases they wish
to present. This also provides me with
an opportunity to discover the current
most pressing dilemmas in clinical practice,
which become the focal points for
the recording.
For this reason, we are about to launch
our first Meet The Professors program* in
colorectal cancer. This format will allow
us to explore the complex biopsychosocial
determinants of critical treatment
decision-making in colorectal oncology,
and hopefully we will learn more about
why in similar patient populations, we
see both consensus and controversy in
treatment recommendations.
— Neil Love, MD
NLove@ResearchToPractice.net
* www.MeetTheProfessors.com
| This survey was developed with Dr Axel Grothey, who also reviewed the findings and discussed these in an interview,
which is excerpted throughout this monograph, along with select comments from other clinical investigators on our Colorectal Cancer Update audio series. The data in this monograph reflect a series of telephone surveys conducted
in August 2005 with fax/email support of randomly selected US-based medical oncologists who spend more than
50 percent of their time in patient care. Sample sizes of 50 to 103 respondents (as noted) are presented. |
Click here to see image
Click here to see image
SELECT PUBLICATIONS
De Gramont A et al. Oxaliplatin/5-FU/LV in
adjuvant colon cancer: Results of the international
randomized MOSAIC trial. Proc ASCO 2003;Abstract 1015.
De Gramont A et al. Oxaliplatin/5FU/LV in
the adjuvant treatment of Stage II and Stage
III colon cancer: Efficacy results with a median
follow-up of 4 years. Proc ASCO 2005;Abstract 3501.
Giantonio BJ et al. High-dose bevacizumab
improves survival when combined with
FOLFOX4 in previously treated advanced
colorectal cancer: Results from the Eastern
Cooperative Oncology Group (ECOG) study
E3200. Proc ASCO 2005;Abstract 2.
Gill S et al. Pooled analysis of fluorouracilbased
adjuvant therapy for stage II and III colon
cancer: Who benefits and by how much? J Clin
Oncol 2004;22(10):1797-806. Abstract
Grothey A, Sargent DJ. FOLFOX for stage II
colon cancer? A commentary on the recent
FDA approval of oxaliplatin for adjuvant
therapy of stage III colon cancer. J Clin Oncol 2005;23(15):3311-3. No abstract available
Hurwitz HI et al. Bevacizumab in combination
with fluorouracil and leucovorin: An active
regimen for first-line metastatic colorectal
cancer. J Clin Oncol 2005;23(15):3502-8. Abstract
Kabbinavar FF et al. Combined analysis of
efficacy: The addition of bevacizumab to
fluorouracil/leucovorin improves survival for
patients with metastatic colorectal cancer. J Clin
Oncol 2005;23(16):3706-12. Abstract
Kelly H, Goldberg RM. Systemic therapy for
me astatic colorectal cancer: Current options,
current evidence. J Clin Oncol 2005;23(20):4553-
60. Abstract
Wolmark N et al. A Phase III trial comparing
FULV to FULV + oxaliplatin in stage II or
III carcinoma of the colon: Results of NSABP
Protocol C-07. Proc ASCO 2005;Abstract 3500.
|

