|
Click here to see image
Click here to see image
Click here to see image
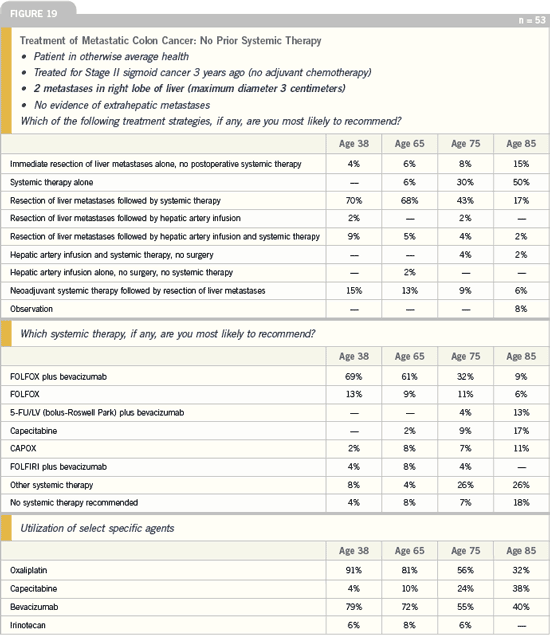
DR LOVE: How would you approach a
patient who presents with synchronous
hepatic and lung metastases (Figure 21)?
DR GROTHEY: This presentation has
a very poor prognosis. What is not an
appropriate choice here is resection of
liver metastases followed by systemic
chemotherapy. This is probably one
area where clinical investigators and
community oncologists differ in opinion.
I would approach this patient with
neoadjuvant therapy followed by resection
of the metastases.
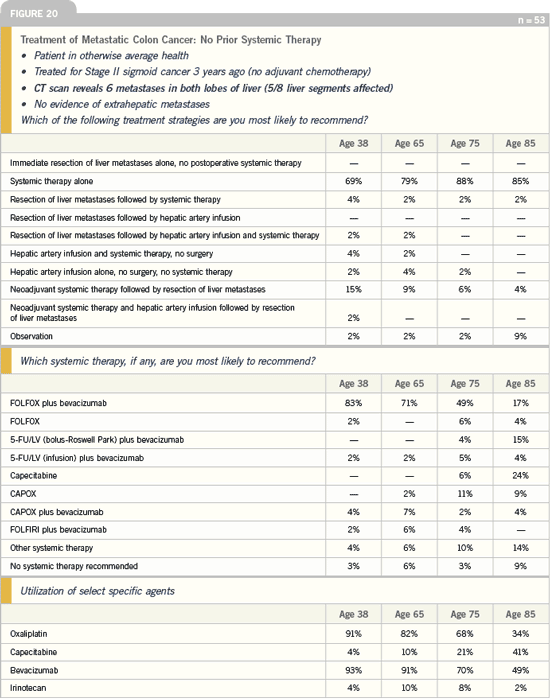
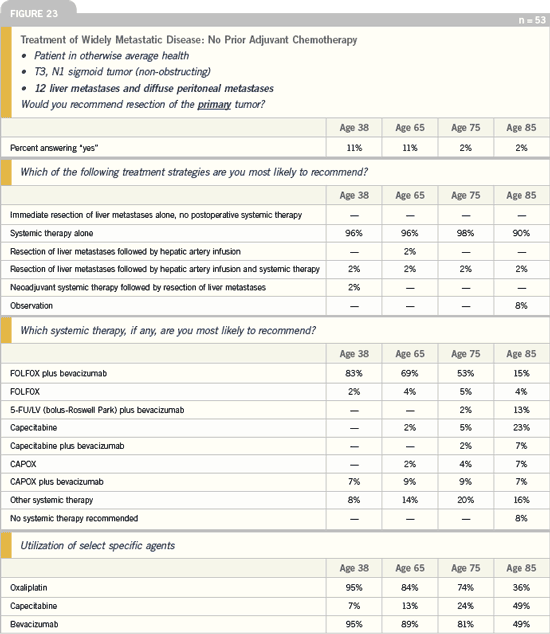
DR LOVE: When you encounter a patient
who has bilateral liver metastases,
presenting with a nonobstructing lesion,
would you resect the primary tumor
(Figure 22)?
DR GROTHEY: I would not resect the
primary tumor, particularly in young
patients. I was a bit surprised in the
shifting with age, because if I had an 85-year-old patient with a nonobstructing
tumor and metastases, I would prefer
to take care of the primary tumor and
prevent any obstruction problem. For
a 38-year-old patient, I would have
clearly said, “No resection of the primary
tumor.” In an 85-year-old, I would probably
resect the primary tumor.
Colorectal Cancer Update 2005 (3) |
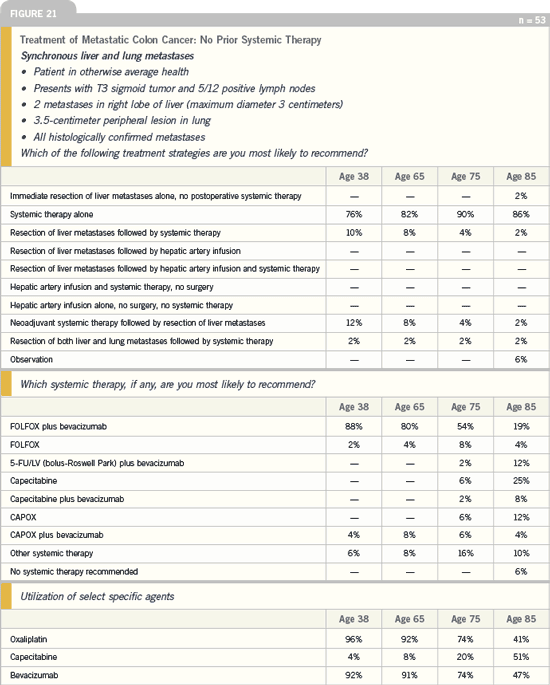
DR LOVE: What treatment strategy
would you recommend for a patient who
presents with metastatic disease and
asymptomatic primary colon cancer?
DR O’CONNELL: We have a study that
has been approved by the National
Cancer Institute, which will evaluate
the need for resection of an asymptomatic
primary colon cancer in patients
who present with metastatic disease.
There’s been a lot of controversy about
this in the literature. Approximately
25 percent of patients with metastatic
colorectal cancer who have an unresected
primary will develop a complication —
primarily obstruction — if that tumor
isn’t resected.
Now that we have more effective systemic
chemotherapy, our goal is to determine
whether we can avoid the need
for resection in patients who don’t have
any symptoms related to the primary
tumor but who have distant, unresectable
metastatic disease. We’ll treat them
all with the modified FOLFOX6 regimen
plus bevacizumab. Our endpoint
of this Phase II trial is to determine the
local complication rates.
Colorectal Cancer Update 2005 (1) |
DR LOVE: Can you talk about the
NSABP trial investigating CAPOX
with or without intra-arterial infusion in
patients with hepatic metastases?
DR WOLMARK: NSABP-C-09 is for
patients with liver-only metastases that
have been removed or ablated. Patients
will receive CAPOX with or without
intra-arterial FUDR. The European
data with CAPOX for patients with
liver-only disease certainly influenced
the decision of the hepatic surgeons to
use CAPOX as the baseline therapy.
The question being tested is the role of
intra-arterial FUDR. I think the real
challenge is to see if hepatic surgeons
from different institutions with different
concepts can work together to develop a
clinical trial.
Colorectal Cancer Update 2005 (2) |
DR LOVE: What systemic therapy regimen
do you generally recommend for a
patient with metastatic colon cancer?
DR VENOOK: At UCSF, we lean toward
FOLFOX rather than CAPOX because
we have data for FOLFOX, and the
current data are not adequate to say that
CAPOX and FOLFOX are equivalent.
In practice we have seen robust responses
with CAPOX, FOLFOX, FOLFIRI and
CAPIRI. Although we need more data,
I do not anticipate that capecitabine will
be a compromise for patients. The problem
we have had with CAPOX has been
dosing, because it can cause hand-foot
syndrome. We are relatively conservative
in our use of capecitabine and tend to
favor it in elderly patients.
Whether research resources should
be invested in investigating capecitabine
in combination with either irinotecan
or oxaliplatin is a good question. On
one hand, with the new agents that need
evaluation, it seems absurd to expend
resources on proving the equivalence
of combinations of capecitabine versus
5-FU. On the other hand, this has a
huge impact on quality of life and patient
satisfaction. In an ideal world, we would
enroll more patients with colorectal cancer
in clinical trials and be able to answer
all of these questions.
Colorectal Cancer Update 2005 (4) |
DR PHILIP: At our institution, we evaluated
the combination of capecitabine
and oxaliplatin (CAPOX). At this time,
our front-line nonprotocol treatment
approach includes bevacizumab and
CAPOX. Granted, no Phase III trial
data are available comparing CAPOX
to FOLFOX.
However, in the metastatic disease
setting, taking into account the convenience
for patients of receiving an oral
agent instead of continuous infusion
5-FU, we feel that CAPOX would be
better than FOLFOX. I probably would
not make the same comment for adjuvant
therapy. But in the metastatic disease
setting, my approach would be bevacizumab
plus CAPOX.
DR LOVE: What are your thoughts
in general, as you evaluate the data in
the survey, in terms of the amount of
CAPOX being recommended?
DR GROTHEY: It is what I would have
expected, although not necessarily what
I would like. I think that the difference
between FOLFOX and CAPOX — in
the absence of Phase III data — is not
so great that FOLFOX should dominate
all of these answers. This is an interesting
phenomenon, particularly when you
look at what is happening across the
Atlantic, where FOLFIRI is relatively
more dominating compared to what we
see here. The split in Europe is 60-
40 or 55-45 in favor of FOLFOX, but
FOLFIRI has substantial market share.
Here, FOLFIRI has a minor share.
I believe that CAPOX is a rational
choice in the metastatic setting. For oxaliplatin in general, I think the standard
of care is clearly a combination
regimen. You would have to make a case
for why you would not be able to use a
combination regimen, particularly since
we know that FOLFOX and CAPOX
regimens are very well tolerated. In fact,
rather than using the Mayo Clinic regimen,
I would rather use FOLFOX,
because it’s better tolerated.
DR LOVE: What about the tolerability of
CAPOX versus FOLFOX?
DR GROTHEY: I think there is no difference
in tolerability once you have determined
the right dose of capecitabine.
My personal preference is for FOLFOX
over CAPOX. This is more or less what
is reflected in this survey. You would
have to make a case as to why you would
utilize CAPOX rather than FOLFOX.
The primary case to be made for
selecting CAPOX over FOLFOX is
convenience. There are patients that I
have put on CAPOX because they
want to travel. They want to be more
independent.
I personally use a lot of capecitabine
in combination with bevacizumab after a
patient can no longer tolerate FOLFOX
because of the oxaliplatin neurotoxicity.
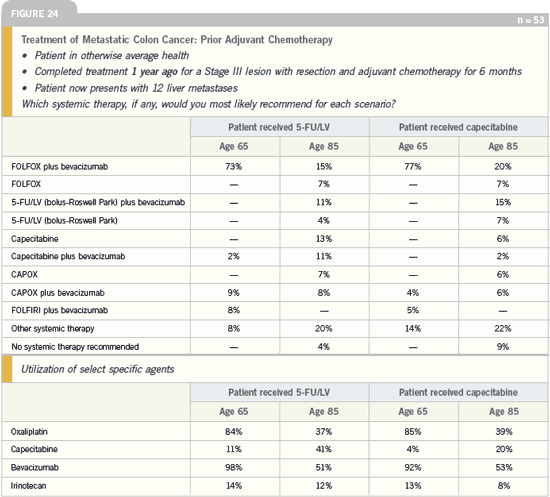
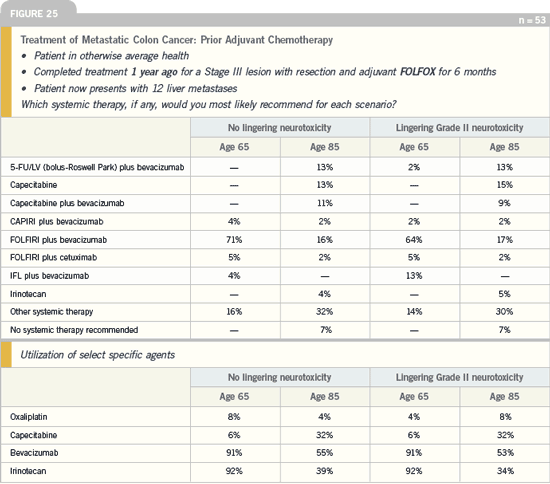
When you look at the patient who was
treated one year ago for a Stage III lesion
with FOLFOX, no one recommended
FOLFOX re-treatment. I would have like
to have seen at least one or two patients
receiving FOLFOX. Interestingly, in
the patient with a Stage III lesion who
received six months of FOLFOX six
months ago with no change in Grade II
neurotoxicity, the same pattern emerges.
Click here to see image
Click here to see image
Click here to see image
Colorectal Cancer Update 2004 (6) |
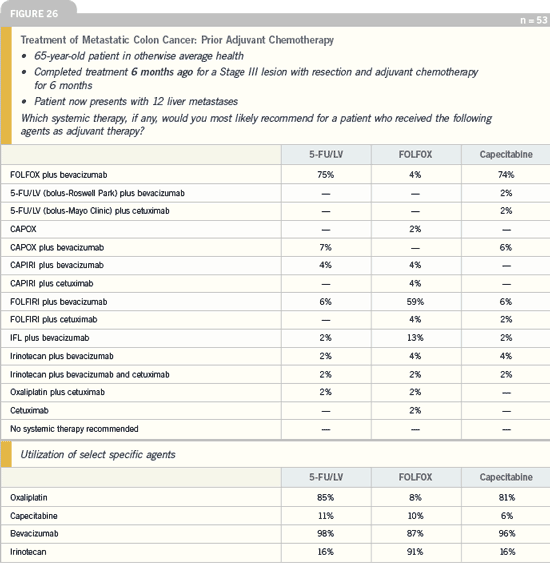
DR LOVE: How do you generally approach the patient who has relapsed after adjuvant
oxaliplatin-based therapy?
DR DANIEL HALLER: My approach to
patients with a colorectal cancer recurrence
after adjuvant therapy depends on
when the relapse occurs. Obviously, this
is now an issue because of the results
from the MOSAIC adjuvant trial. In a
patient who relapses less than six months
after adjuvant FOLFOX and still has
neuropathy, I would use FOLFIRI plus
bevacizumab as first-line therapy. That
type of patient would need all the help
available, not sequential therapy.
A patient who relapses after adjuvant
FOLFOX and doesn’t have neuropathy
could be treated as a “virgin patient,”
and whichever chemotherapy regimen is
best for that patient should be selected,
independent of their adjuvant therapy. In
those situations, I base my chemotherapy
decision on the Tournigand data. Then
I select the most tolerable and efficacious
biologic agent and marry it to the
chemotherapeutic regimen that is best
for the patient.
The best regimen is dependent
upon both its efficacy and toxicity.
For example, the first violinist in the
Philadelphia Orchestra might be treated
with FOLFIRI plus bevacizumab. In
my clinic, patients will not be treated
with IFL plus bevacizumab; they also
won’t be treated with capecitabine plus
bevacizumab outside of a trial. For the
nonviolinist, most often FOLFOX plus
bevacizumab would be selected.
In certain patients, bolus 5-FU/leucovorin
— the Roswell Park regimen
— plus bevacizumab, as used in the trial
by Kabbinavar and one of the arms of
the trial by Hurwitz, would be a reasonable
option. Based on the data from
the trial by Hurwitz, the efficacy of the Roswell Park regimen plus bevacizumab
is somewhere in between the efficacy for
IFL alone and IFL plus bevacizumab. I
believe the Roswell Park regimen plus
bevacizumab is probably less efficacious
than FOLFOX plus bevacizumab.
Click here to see image
Click here to see image
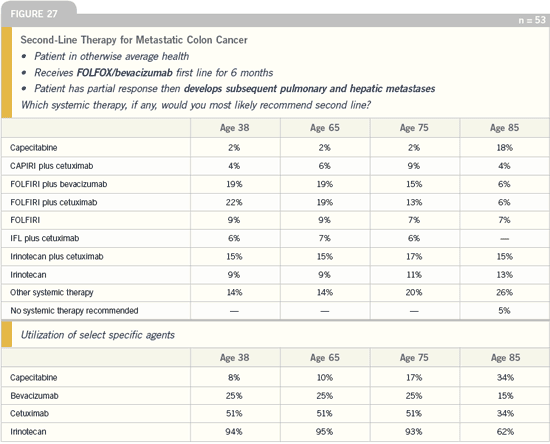
DR LOVE: What is your take on the
treatment choices in the survey for the
patient with colon cancer who progresses
on FOLFOX/bevacizumab?
DR GROTHEY: I would not have expected
50 percent of the physicians to select
cetuximab — whether or not it is with
FOLFIRI. I would have anticipated
something in the range of approximately
20 percent. This is not an approved regimen
in this situation and it is clearly
off label. It is interesting to see that
25 percent of the patients continue to
receive bevacizumab.
The idea is that with bevacizumab,
you enhance the activity of chemotherapy,
regardless of the type of chemotherapy.
You target genetically stable endothelial cells and increase the delivery
of chemotherapy into the tumor, where
you have the anti-angiogenic effect.
Click here to see image
Cetuximab has the same overall
effect, but it is expensive and it is a
last-line indication. So you can use it
as a later option, particularly after second-
line bevacizumab, which works in
patients who have not previously received
bevacizumab.
The continuation of bevacizumab in
second line is really interesting. I believe
that a continuation of bevacizumab is
logical. However, FOLFOX/bevacizumab
followed by FOLFIRI followed
by irinotecan/cetuximab is perhaps the
best-established sequence at present.
I personally continue bevacizumab
because of the idea that it works on normal,
genetically stable cells. My hypothesis
is that the resistance we observe
with FOLFOX/bevacizumab as firstline
therapy is to FOLFOX, not to bevacizumab.
Bevacizumab enhances the
activity of chemotherapy; in colorectal
cancer, it has been shown for 5-FU,
irinotecan, cetuximab and oxaliplatin.
As we’re targeting genetically stable
endothelial cells that provide neovascularization
to the tumor, I think it
definitely makes sense to use it this way.
The role of bevacizumab following disease
progression, however, is unclear.
This is the main reason SWOG and
NCCTG will be conducting a trial, the
Intergroup Bevacizumab Continuation
trial, in which patients who have progressed
on FOLFOX/bevacizumab or
FOLFOX followed by 5-FU/leucovorin/
bevacizumab will be randomly assigned
to additional therapy with or without
bevacizumab.
Click here to see image
Click here to see image
Colorectal Cancer Update 2005 (1) |
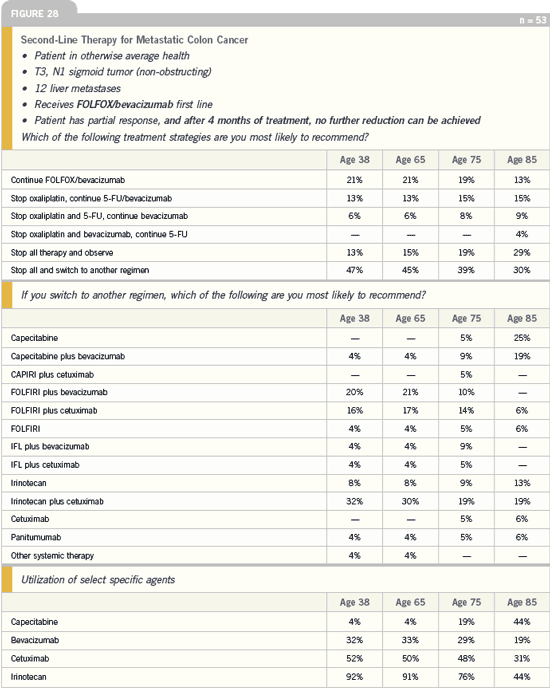
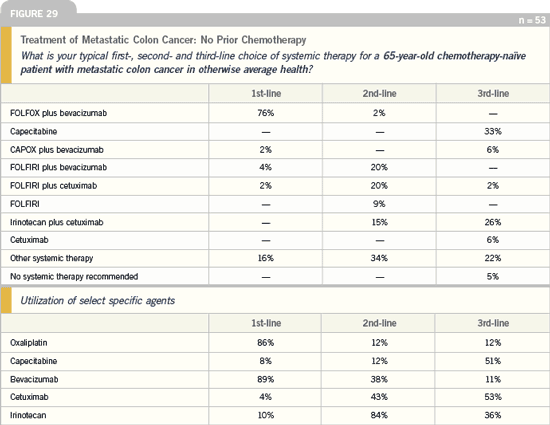
DR LOVE: What is your general treatment
algorithm for a patient with metastatic
colon cancer?
DR HOWARD HOCHSTER: In a clinical
setting, I’ve been comfortable using an
oxaliplatin-based regimen in combination
with bevacizumab as first-line therapy
for patients with metastatic disease,
based on the TREE study and our own personal experience. The best data for
improved time to progression, response
rate and survival are with bevacizumab
as first-line therapy, and I am most
comfortable using oxaliplatin in the firstline
setting. Therefore, I tend to use
FOLFOX with bevacizumab in patients
not enrolled on a protocol. We have seen
nice responses and patients staying on
those regimens for a long time.
Irinotecan and cetuximab would be
very reasonable second-line options,
whether it’s with single-agent irinotecan
and adding in cetuximab at the time of
progression or, taking out the reimbursement
issues, starting with both cetuximab
and irinotecan together, which
would make sense.
The third-line setting is wide open,
and clinical trials would definitely have a
value in identifying new agents.
Colorectal Cancer Update 2005 (4) |
DR PHILIP: For patients with disease that
has progressed on an oxaliplatin-based
treatment, we move to an irinotecanbased
therapy. The question becomes,
Do we use irinotecan as a single agent or
in combination with a fluoropyrimidine
(eg, capecitabine or 5-FU/leucovorin)?
The third- or fourth-line options would
be any of these agents with or without
cetuximab.
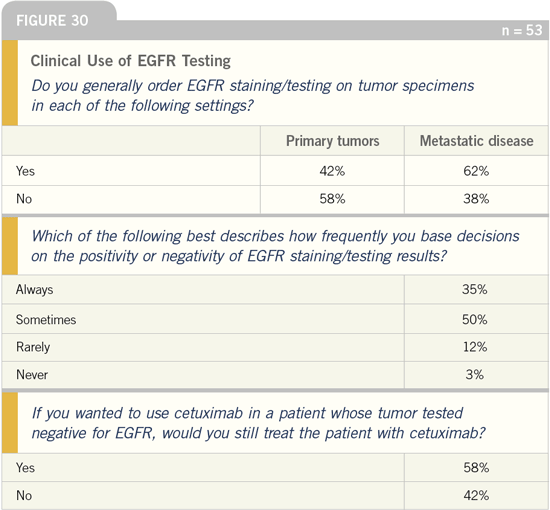
DR LOVE: According to the physicians’
responses, a substantial number of doctors
continue to order EGFR testing
(Figure 30). Why do you think this
is occurring?
DR GROTHEY: I believe that a number
of physicians request EGFR testing to
avoid the hassle of communicating with
the insurance company. If the test results
are positive, there is no need to worry
about reimbursement issues. However,
if the results are negative, the doctor
has to struggle to make a case to utilize
cetuximab. We need to translate to the
community oncologists that this test is
not really necessary.
Another interesting question is, Do
you test in the primary tumor for EGFR
in colon cancer? In breast cancer patients,
we automatically determine the hormone
receptor status of the primary tumor.
For the colon cancer patient, testing the
primary tumor for EGFR positivity is
not needed. If you utilize cetuximab,
it’s better to use it with irinotecan (Figure 31). Although it is logical to
say, “If you have first-line oxaliplatin,
you don’t necessarily need cetuximab”
because it is not approved and it is expensive.
Additionally, it is not as beneficial
in a salvage therapy setting.
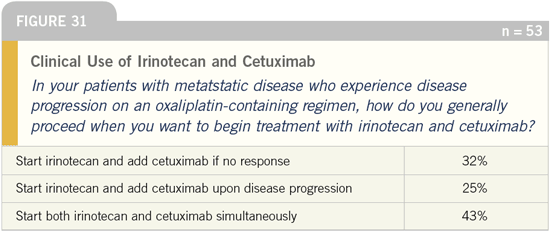
Colorectal Cancer Update 2005 (3) |
DR LOVE: Should the decision whether
or not to administer cetuximab be based
on EGFR testing/staining results?
DR SALTZ: We published an article in the
Journal of Clinical Oncology that reports
activity with cetuximab in colorectal
cancer in tumors that do not express
the EGFR by immunohistochemistry
(IHC).
These are very compelling data. We
all wanted to believe that EGFR would
be an important prognostic indicator,
but our technology for assessing EGFR
expression is flawed.
We generally use the primary tumor
as the basis for the EGFR status of the
metastasis, but that appears to be inaccurate.
Data show that EGFR degrades
over time.
At this time, no clinical decision
should be made on the basis of EGFR
staining. Specifically, no patient should
be excluded from a therapy — cetuximab
or otherwise — simply because
their IHC staining for EGFR is negative
and, just as importantly, no patient
should be treated with these agents
simply because the tumor is strongly
EGFR positive.
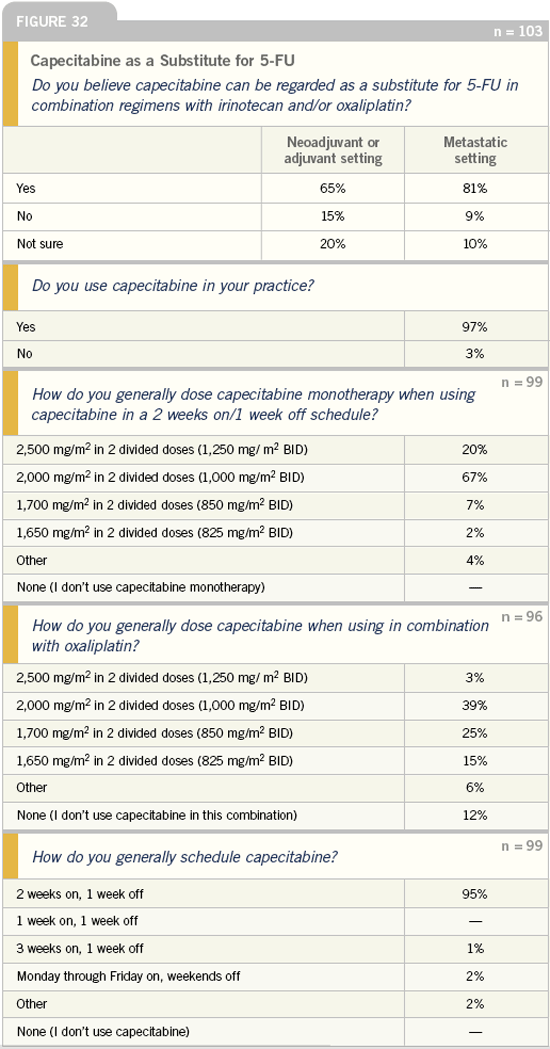
DR LOVE: The number of physicians who
believe that capecitabine can be regarded
as equivalent to 5-FU in the neoadjuvant,
adjuvant and metastatic settings is pretty
intriguing. It appears as though they
are influenced by the results of the
X-ACT trial. What is your interpretation
of this?
DR GROTHEY: They may believe that
capecitabine is a substitute for 5-FU
in these settings; however, the dosing
of capecitabine has not yet been well
defined in the United States. A large
trial in patients with colon cancer is
being conducted, which utilizes a dose of
1,000 mg/m2 twice a day in a one week
on, one week off schedule.
Personally, in the adjuvant setting, I
start with a dose of 2,500 mg/m2 in two
divided doses and then decrease as needed.
In the metastatic setting, I prefer to
start with 2,000 mg/m2 in two divided
doses. When dosing capecitabine with
oxaliplatin, I utilize 850 mg/m2 twice a
day, based on the American experience.
Colorectal Cancer Update 2005 (3) |
DR ROBERT WOLFF: The maximum dose
of capecitabine that I use as a single agent
is 2,000 mg/m2 in two divided doses.
If I combine capecitabine with another
agent, including irinotecan, oxaliplatin,
or radiation, the dose is decreased to
the 1,500 to 1,800 mg/m2 range. If a
patient experiences toxicity, the dose is
reduced accordingly.
Colorectal Cancer Update 2004 (5) |
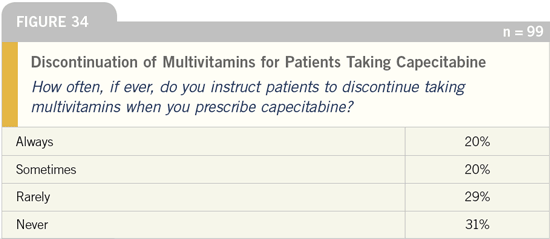
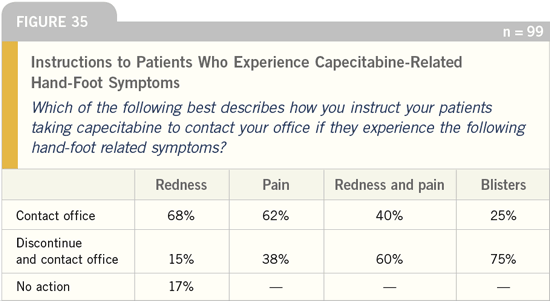
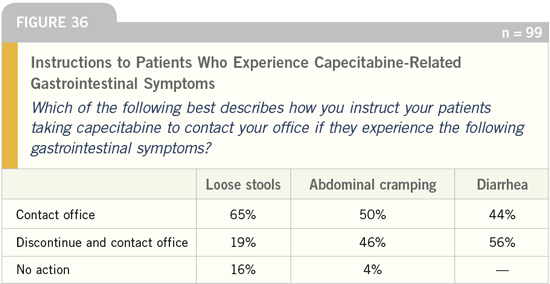
DR LOVE: What action do you instruct
your patients to take if they experience
capecitabine-related toxicity?
DR CASSIDY: We make an effort to
educate patients about the potential
for diarrhea because if patients develop
diarrhea, they may become dehydrated
and require hospitalization. Sometimes
diarrhea is associated with neutropenia.
Diarrhea and neutropenia together
are dreaded side effects of the fluoro
pyrimidines.
I tell my patients they should stop
treatment and inform us if they are having
diarrhea more than five times in a
24-hour period.
Hand-foot syndrome is a bit more
subtle. Patients often develop a minor
degree of hand-foot syndrome with the
first cycle of capecitabine, and it may
be worse with the second cycle. At that
point, we reduce the capecitabine dose.
Because of that strategy, I don’t see
many patients with severe hand-foot syndrome.
We also tell patients to stop
treatment if they develop redness of their
hands or feet with pain that interrupts
their level of functioning.

Select publications
|

