| |
Adjuvant Systemic Therapy |

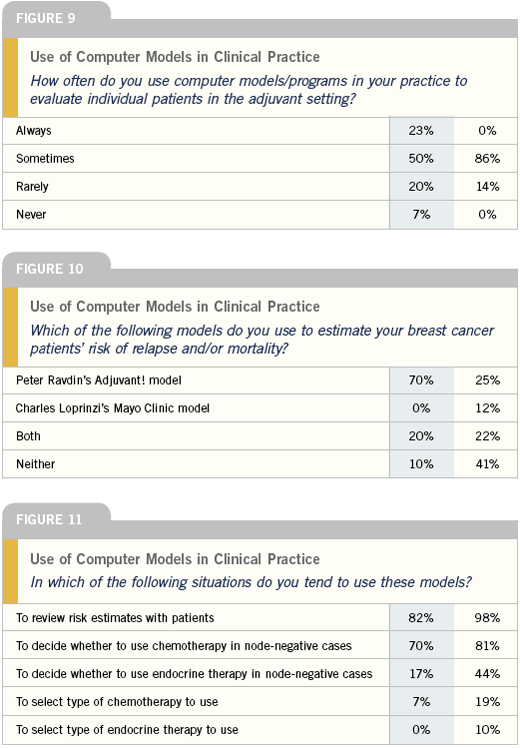
Computerized risk models
Peter Ravdin and I did a lot of this work together, and we published a paper several years ago in the Journal of the NCCN, which compared and contrasted the two tools. These programs are becoming increasingly established among community oncologists. Peter’s program Adjuvant! has been validated against the natural experience of the British Columbia Group, which was presented in abstract form at ASCO 2004.
The advantage to having both tools out there is that it allows some honesty between the people developing them and keeping them updated. The Mayo Clinic program is quite user-friendly, but you could also argue that it doesn’t have as much flexibility and as many nuances as Adjuvant!.
— Charles L Loprinzi, MD
My group is very involved in developing and validating risk models from a methodological, statistical standpoint because these models are very easy to produce, but not easy to produce right. The one model that’s captured everybody’s imagination more than any other is Peter Ravdin’s Adjuvant! program. We use Adjuvant! virtually on a daily basis.
What is often misunderstood about these models is that the estimates they generate —while probably better than what we’ve had in the past — are still estimates. They’re based on extrapolations often from highly selected patients put through very formal clinical research trials. In some cases they can be extrapolated to the general population or to patients who wouldn’t have been in those trials. Peter has attempted to adjust for co-morbidities, but the adjustment there is pretty crude and broad and has to be interpreted by the patient’s treating oncologist.
— Gary Lyman, MD, MPH
Click here to see image
I have book marked both the Mayo Clinic and the Adjuvant! Online program in all the clinic’s computers. I’m using Adjuvant! Online most commonly, especially when I think a patient could really benefit from seeing some numbers. Sometimes I need some help sorting out exactly what added risk reduction would be predicted from the data, for example in adding chemotherapy to hormone therapy — I’m always looking for support not to give chemotherapy.
— Julie Gralow, MD
Adjuvant chemotherapy for node-negative disease
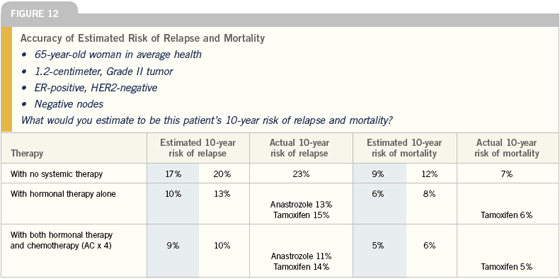
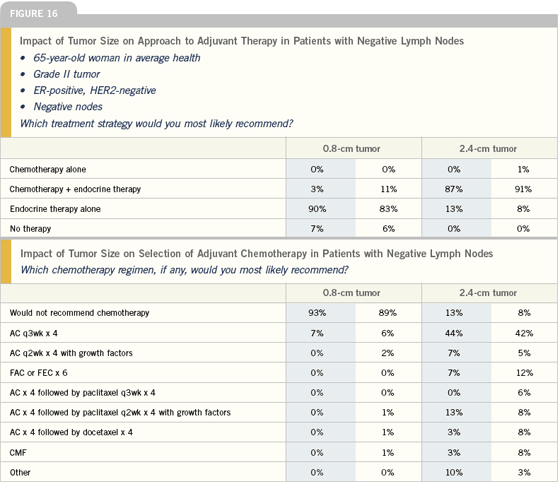
Ultimately, most women with node-negative, ER-positive disease who go to see an oncologist are asking: “What would chemotherapy add to my care?” A number of parameters need to be factored into this discussion, including tumor size, HER2 status, tumor grade in some cases, the presence of lymphovascular invasion and, of course, the patient’s age and comorbidities.
All of these elements are considered, but usually it comes down to a discussion of whether chemotherapy adds enough to standard care — usually a hormonal manipulation — to justify the added toxicity. This is a situation where an aid like Adjuvant! is helpful. You can use the results from the model to say to a patient, “Here’s your risk with or without adjuvant chemotherapy and here’s your risk with or without adjuvant hormonal therapy.”
I also feel very strongly that we need to incorporate the patient’s value system into the discussion and the final decision. What I believe is a reasonable benefit needed to accept the toxicity from a chemotherapy regimen may be very different from what a patient views as reasonable, particularly when we are talking about just a few percentage points.
— Gary Lyman, MD, MPH
My current approach to patients with ER-positive, node-negative disease is to look at tumor size and other prognostic factors to decide whether or not to add chemotherapy to hormone therapy. I foresee that in the next six to 12 months, I will be using the Genomic Health information in making those decisions for some of these patients.
Right now, I’m not using that information in my practice because of logistical issues such as reimbursement, technology acceptance and turnaround time.
I factor the patient’s age into my choice of hormonal therapy and whether or not to use chemotherapy. With a young woman in a node-negative setting, I’m much more likely to use chemotherapy, and I may go with four cycles of AC or even add paclitaxel to AC with a larger or high-grade tumor.
In elderly patients, I think the Adjuvant! Online program performs very well because it allows you to take into account age and their co-morbidities in determining the impact of treatment. So, these are patients that I am likely to plug into Adjuvant! to get a handle on the impact of the disease and treatment.
— Generosa Grana, MD
I try very hard not to give chemotherapy to patients with ER-positive, node-negative disease. But there are things like tumor size (over a centimeter, especially if it’s high grade) HER2-positivity, lymphovascular invasion or other features that may push me to give chemotherapy. I think the preponderance of data to date shows us that people who benefit the most from chemotherapy are those with higher-risk tumors, which tend to be the ER-negative or HER2-positive. That’s not to say that people with ER-positive or HER2-negative cancers don’t benefit from chemotherapy, but rather they seem to benefit less. So for me, it’s really a matter of weighing the risks and benefits, including the risk of the cancer itself.
— Ann Partridge, MD
Click here to see image
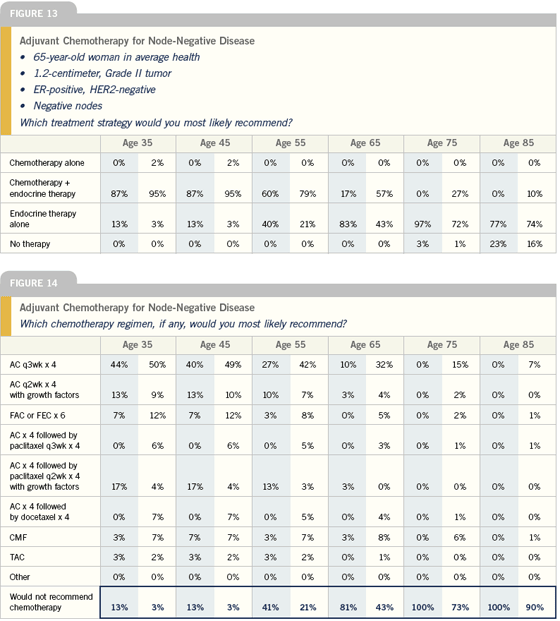
For adjuvant chemotherapy in the lowerrisk, node-negative setting, I generally use four cycles of AC. The controversial issue at this point is whether to use the traditional every three-week schedule or dose-dense therapy with hematopoietic growth factor support.
Dose-dense schedules are intriguing in that they are somewhat better tolerated because of the growth factors and the patient finishes therapy faster. They come with, of course, a great deal of additional cost.
Most importantly, however, we probably could benefit from additional validation that AC given every two weeks has an advantage over an every three-week administration. Clearly, dose-dense AC paclitaxel showed an advantage in CALGB-9741 that most oncologists have accepted. But whether we can convert that benefit to a lower-risk, node-negative setting with AC times four alone is controversial.
In my own practice, I discuss with patients the benefits of quicker therapy, the downside in terms of additional injections and cost, and the uncertainty regarding the additional benefit of dosedense AC. I’m very comfortable, however, if a patient chooses to go that route, that we’re not doing her any harm.
— Gary Lyman, MD, MPH
Click here to see image
ATAC trial update
The ATAC trial has reached an important point in its evolution with a median follow-up of 68 months. Almost all of the patients are now off therapy, and we have one year of follow-up after the therapy is completed.
The progress of this trial is important for two reasons: it makes me comfortable about the efficacy and the hypothetical “carry-over effect” we’ve seen with tamoxifen, and I’m also more comfortable with the toxicities and tolerability of anastrozole.
I believe this is probably the most important of the three ATAC analyses, and it allows me, as a practicing clinician, to change practice. I speak not only as a practicing clinician but also as the past principal investigator of the trial.
The simplest interpretation of the results is that anastrozole prevents one in four of the relapses we see in postmenopausal patients on tamoxifen. That translates into highly significant improvements in disease-free survival, recurrence-free survival and distant disease-free survival.
The absolute number for difference in recurrence-free survival in the patients with receptor-positive disease at six years is close to four percent. It is important to remember that this trial included a group of patients with a relatively good prognosis.
In terms of relative risk reductions, we have no reason to suppose that the relative risk reductions will be different in any subgroup, and if that one in four relative risk reduction is across the board, then in a subgroup of patients with, for example, a 40 percent chance of relapse at six years, the absolute reduction is about 10 percent, not four percent, as was seen in the ATAC trial.
— Michael Baum, MD, ChM
Click here to see image
The use of anastrozole instead of tamoxifen does not impair quality of life. We can also say, with confidence, that the gynecological symptoms linked to tamoxifen have now translated into a fourfold increase in hysterectomy rates with tamoxifen compared to anastrozole.
That is a dramatic observation, which we nearly missed. I was persistent about tracking down all the hysterectomies in women who had their wombs at the time of randomization. We came up with an extraordinary figure — I believe it’s the most extreme relative risk I’ve encountered in clinical trials.
The absolute numbers of hysterectomy were 1.3 percent versus 5.1 percent for anastrozole and tamoxifen, respectively. This has a profound economic impact. I also don’t know how many hysteroscopies are being performed for every hysterectomy or how much the workup costs to decide whether a woman should have a hysterectomy, but these are big cost issues.
The update doesn’t give us any new information with regard to other prespecified adverse events, and no other adverse event is emerging with a frequency of more than one percent.
The fracture rate incidence is becoming a little more reassuring. An excess fracture rate occurs in the first two or three years, but then the lines are beginning to come together. As patients stop taking anastrozole, the fracture rate returns to that of the patients randomized to tamoxifen.
Thus, so far, no difference has occurred in fractures of the neck or femur, which are of particular concern. I think the issue of bone is easy to manage. We should monitor bone mineral density, perhaps exclude patients who have established osteoporosis, and then be ready to intervene with a bisphosphonate when the patient becomes osteopenic.
The polyarthralgia with anastrozole remains a problem. We don’t understand it, and it occasionally leads to withdrawal of treatment; however, the bottom line is that a significant difference exists favoring anastrozole for patients withdrawing from treatment because of side effects. If you evaluate the totality of side effects, anastrozole does better. If you consider the issue of the gynecological symptoms leading to hysterectomy, I believe the new drug — anastrozole — has the better tolerability profile.
— Michael Baum, MD, ChM
Adjuvant endocrine therapy in postmenopausal women
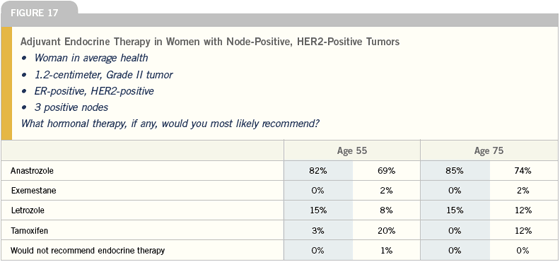
Five major studies examining three different patient populations have reported on aromatase inhibitors in the adjuvant setting. First are the studies of initial therapy comparing tamoxifen versus an aromatase inhibitor. This includes the ATAC trial with anastrozole and the BIG-01-98 trial with letrozole.
The sequential therapy trials examine patients who have been on adjuvant tamoxifen for two to three years and are then randomly assigned to continue tamoxifen or switch to an aromatase inhibitor. The three trials that have been reported are the Intergroup study with exemestane, the ARNO trial with anastrozole and a smaller study with anastrozole presented by Boccardo.
Click here to see image
The extended endocrine therapy trial, MA17, evaluated patients who had taken tamoxifen for four-and-a-half to six years and were randomly assigned to no further endocrine therapy versus switching to letrozole.
It’s important to point out that none of these strategies have been compared head-to-head, so we don’t know whether it would be best to use aromatase inhibitors up front, after two to three years or after five years of tamoxifen. We also don’t know the optimal duration of treatment with aromatase inhibitors.
However, the trend in all of these trials has been that women who received an aromatase inhibitor had a lower risk of disease recurrence than women who remained on tamoxifen or, as in MA17, received no further endocrine therapy. That led the ASCO technology assessment panel to recommend that postmenopausal women with hormone receptor-positive breast cancer should receive an aromatase inhibitor at some point in their treatment.
Despite the major questions that remain — including scheduling and duration of treatment — clearly, aromatase inhibitors have a role in treating postmenopausal women with breast cancer.
— Harold J Burstein MD, PhD
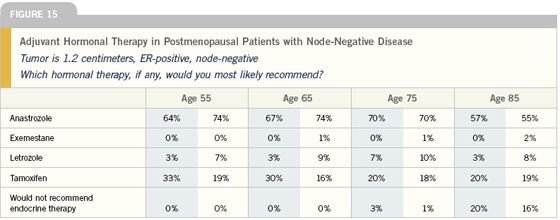
We have two studies using an aromatase inhibitor up front — ATAC with anastrozole and BIG 1-98 with letrozole; however, we have more data on anastrozole with more than five years of follow-up. There doesn’t appear to be any difference, so far, in efficacy, so I would use anastrozole off study because of the toxicity profiles.
I believe that one should use an aromatase inhibitor after two to three years of tamoxifen because of the IES and ARNO/ABCSG data. The ARNO/ABCSG trial with anastrozole is a good study with 1,600 patients in each arm, and if you compare the data to the IES study, the agents are very similar in terms of efficacy. The hazard ratio for relapse-free survival in the IES study was 0.68 and in the ARNO study it was 0.59. I would utilize exemestane, but I believe these two agents are equivalent, and we now have data to support either anastrozole or exemestane after two or three years of tamoxifen.
After five years of tamoxifen, we have only the MA17 trial, so I believe letrozole should be used in this setting. However, if the cardiac concerns continue and they are confined to exemestane and letrozole, that may change my view.
— Anthony Howell, MD
The data from the ATAC trial and the BIG-FEMTA trial are very difficult to compare for a number of reasons. There are different numbers of patients with positive nodes in the two studies, and different percentages who received chemotherapy. Another point is that the BIG-FEMTA is a short-term analysis of data at the present time. For the core group, the followup is only 25.8 months, whereas the current data for anastrozole are beyond five years.
The other concern about the letrozole data is the high incidence of hypercholesterolemia and greater number of cardiac deaths, despite small numbers of events. We also saw excessive cardiac events with exemestane in the IES study.
The data from ATAC on cardiac events will be presented this year, but if letrozole is more potent — and I think it is — you may not want the most potent drug in the adjuvant setting, because it may have more adverse events. We clearly need longer-term data before we start using letrozole upfront for five years.
At this point, in terms of optimal hormonal therapy for ER-positive, postmenopausal patients, the current data indicate that for most women, anastrozole should probably be first line. I think we need more data on letrozole.
— J Michael Dixon, MD
It’s very exciting that we’ve got lots of choices now in hormonal treatments and several categories of very active drugs with very good toxicity profiles. But it’s confusing to know exactly what to do in terms of our postmenopausal, hormone receptor-positive women. Should we start with an AI? Should we give tamoxifen for two years or five years, if we’re giving it at all?
I do agree with the ASCO Tech Assessment that says that for the majority of postmenopausal, hormone receptor- positive women, at some point an adjuvant AI is absolutely indicated. But I’m not starting everybody, up front, on an AI.
If a patient has strongly ER/PR-positive, HER2-negative disease and no history of clotting, for example, and if she has osteoporosis or hyperlipidemia, I would probably start her on tamoxifen. Whereas if a patient has a PR-negative or HER2-positive tumor, settings where there’s at least some hints that maybe the AI’s are clearly superior, then I’m going to start the patient on an up-front AI. I still discuss both tamoxifen and the AIs with my patients.
— Julie Gralow, MD
In the past, adjuvant endocrine treatment in postmenopausal women was really easy, because everyone was treated with tamoxifen. Today the decision is fairly complicated because you need to consider individual patient issues and factors, like cost, risk for heart disease and strokes and risk of the disease itself. A patient with a tiny node-negative cancer isn’t going to derive much benefit from anastrozole compared to tamoxifen, whereas higher-risk patients with multiple positive nodes should probably be treated with an aromatase inhibitor from the beginning. Generally, that is my preference unless the patient is intolerant or refuses.
So I certainly would consider an aromatase inhibitor up front, and we have a choice of two now — anastrozole and letrozole — with the most data on anastrozole. Starting with an aromatase inhibitor is a proven treatment option, but it’s not the only one. Another option would be to treat with tamoxifen for a couple of years and then switch to an aromatase inhibitor. In US Oncology, we’re about to reopen a trial investigating those two treatment options; an aromatase inhibitor from the beginning versus a couple of years of tamoxifen followed by an aromatase inhibitor. According to the ASCO tech assessment on aromatase inhibitors, both options are very reasonable.
The argument against initial treatment with tamoxifen and switching to an aromatase inhibitor is the chance you will lose patients between day one and year two by not putting them on an aromatase inhibitor. For the patients who relapse in the first six to 12 months, I don’t believe the choice of endocrine treatment will make a difference. There is probably a point where it will begin to make a difference if you don’t switch from tamoxifen to an aromatase inhibitor, which seems to be around two years.
— Stephen E Jones, MD
Management of premenopausal women to maintain fertility
For premenopausal women with ER-negative disease interested in preserving fertility, there is currently a study being run through SWOG evaluating whether or not ovarian suppression can protect ovarian function through chemotherapy. If the woman has ER-positive disease and is not a candidate for that study, I would talk to her about the availability of leuprolide acetate and the lack of data that it actually works, but that it might. I’d prefer to do it on study, but if a person wants everything, I’d say okay.
First and foremost though, I would think about the need for chemotherapy and how much the benefits for the cancer compare to the risks of becoming infertile. I would look at age and think about which treatment to give a patient.
I would lean more towards AC, certainly more than CMF, because oral cyclophosphamide-containing regimens are much more likely to cause people to go into premature menopause. I would also try to stay away from the longer regimens with much more cyclophosphamide such as CEF or CAF compared to AC. I also sometimes send patients to a reproductive endocrinologist, if it’s something that’s very important to them and/ or they have a high likelihood of going through menopause with treatment.
— Ann Partridge, MD
We see many younger patients who wish to preserve their fertility, as we have a very active research program for embryo preservation prior to starting chemotherapy.
Our recommendation for patients in the adjuvant setting is very age-dependent. We generally recommend chemotherapy to most of our very young patients (30 or under), and we usually counsel them that the chemotherapy probably won’t affect their fertility. We advise our patients who are 40 or over that the chemotherapy probably will affect their fertility. Generally, we speak to the women in the 30 to 40 range about our research program in embryo preservation prior to chemotherapy.
— Anne Moore, MD
Adjuvant endocrine therapy in premenopausal patients
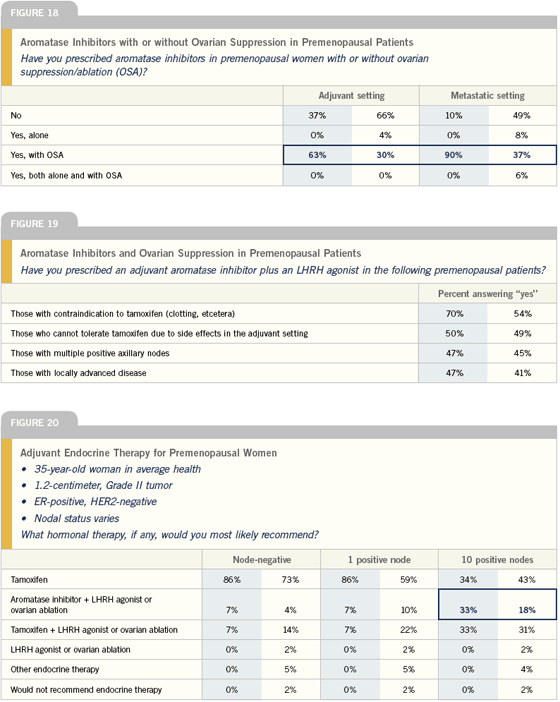
In premenopausal patients, the endocrine therapy of choice for adjuvant therapy is still tamoxifen. In select high-risk patients – those very young patients with many positive nodes where chemotherapy has not rendered them menopausal, I do consider ovarian suppression.
If the chemotherapy has put the patient in limbo for a while, I would treat for a couple of years with tamoxifen, checking periodically to confirm — by low estradiol levels and high FSH levels — that they really are postmenopausal, then switch to an aromatase inhibitor.
In the higher-risk premenopausal patient, where I consider ovarian suppression, I would probably use an aromatase inhibitor along with ovarian suppression — although there is no data to support this approach. Two studies indicate that aromatase inhibitors are better than tamoxifen up front, so I’m going to pick one of those agents, and I would probably go with anastrozole because there’s more data.
— Stephen E Jones, MD
Click here to see image
Click here to see image
There is tremendous interest in the role of aromatase inhibitors with ovarian ablation or suppression in premenopausal women in the adjuvant setting. It is a very exciting possibility for the future, and I’m willing to put patients on clinical trials addressing this, but I am unwilling to apply this to clinical practice until I have some clarification on the true benefit and risk. We have no data on the ultimate effectiveness of that strategy, and we do know there is a significant price to be paid in terms of bone, based on Dr Gnant’s data.
In premenopausal women, I tend to use tamoxifen alone for low-risk disease and tamoxifen and chemotherapy for higher-risk disease. I will use ovarian suppression and tamoxifen for women who don’t want chemotherapy and for those who continue to menstruate following chemotherapy.
— Generosa Grana, MD
Aromatase inhibitors are contraindicated in premenopausal women because they don’t work in this population. Aromatase inhibitors suppress nonovarian sources of the aromatase enzyme and, if the woman has a functional ovarian reserve, then when exposed to an aromatase inhibitor, her ovary makes more aromatase enzyme to overcome that effect.
My colleagues at Dana-Farber and MD Anderson and I have been collecting a series of women who were premenopausal when given aromatase inhibitors inappropriately. Typically, these are women who had chemotherapy-induced amenorrhea, began an aromatase inhibitor and then, anywhere from six to 36 months later, began to menstruate again, including one woman who became pregnant while on aromatase inhibitors. These agents are not at all appropriate for premenopausal women.
The importance of ovarian suppression in premenopausal patients is unknown and is not widely utilized in the United States. It’s more commonly used in Europe. Some very important trials of ovarian suppression are ongoing, most notably the SOFT trial, which is a randomized study of tamoxifen alone versus tamoxifen plus ovarian suppression versus an aromatase inhibitor plus ovarian suppression as primary endocrine therapy for premenopausal women.
Click here to see image
The SOFT trial is being conducted by the IBCSG, and I am involved in measuring the quality of life in that study. It’s a very important trial that will define whether ovarian suppression is essential.
At the present time, tamoxifen is the standard for adjuvant therapy in premenopausal women. While the benefit of adding ovarian suppression is uncertain, it clearly accelerates side effects such as hot flashes, osteoporosis, vaginal dryness and sexual dysfunction.
— Harold J Burstein MD, PhD
For adjuvant systemic therapy in premenopausal women with early stage disease, we generally use tamoxifen alone or with chemotherapy. Sometimes we also use tamoxifen plus ovarian suppression instead of chemotherapy.
Part of this decision is related to patient preference. In a patient with lowrisk disease for whom the chemotherapy is going to provide a very small benefit, I’m perfectly comfortable using ovarian suppression for two years as a substitute for chemotherapy.
— Anne Moore, MD
Chemotherapy for patients with node-positive disease
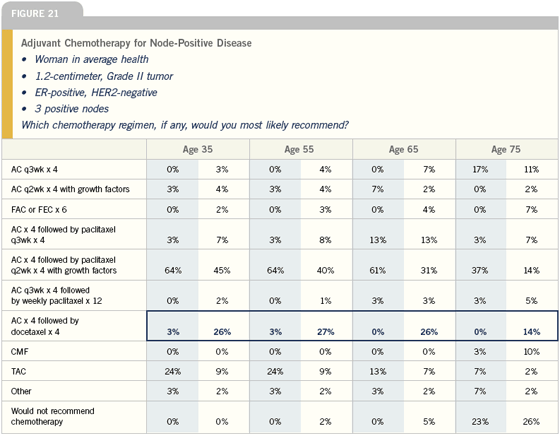
For patients with ER-positive disease and multiple nodes positive, I usually use AC with or without a taxane — often dose dense. As we learn more about the biology of these diseases and separate out the cancers by more than just ER-positive and ER-negative, I hope that we can give fewer people chemotherapy.
— Ann Partridge, MD
The situation sometimes arises in older patients with high-risk disease, in whom you may not want to utilize adjuvant chemotherapy. I received an email about an 83-year-old woman with an ER/PRnegative tumor and three positive nodes from a physician who felt a great deal of pressure to use chemotherapy. I think about the only thing that can happen there is a disaster. There are patients in whom the age and general condition just does not permit the use of adjuvant chemotherapy. In these patients, you’ve got to accept that and move on. Our first goal is to do no harm.
Having said that, adjuvant chemotherapy is an individual decision. I certainly have comfortably used chemotherapy in women up into their seventies. In these women, I’m much more likely, regardless of the nodal status, to use AC times four. AC  paclitaxel is also pretty well tolerated in the older group of women. paclitaxel is also pretty well tolerated in the older group of women.
In younger women, we have a number of different choices for adjuvant chemotherapy. I am not a dose-dense person. We have one study demonstrating a fairly small difference, and in Don Berry’s presentation in San Antonio, we saw that dose-dense therapy didn’t seem to work in the ER-positive group. Almost all of the treatment effect was in the ER-negative subset. So in my practice, I wouldn’t consider dose-dense chemotherapy in a patient with ER-positive disease.
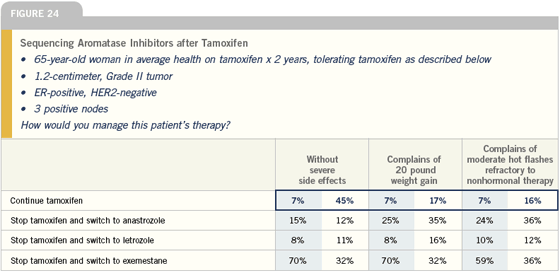
Click here to see image
I do think AC 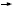 docetaxel — the control arm in our current US Oncology study — is a very reasonable treatment that doesn’t require growth factors. TAC would also be an option. TAC does require growth factors but has about the same treatment duration as dose-dense therapy, and I would use this regimen. docetaxel — the control arm in our current US Oncology study — is a very reasonable treatment that doesn’t require growth factors. TAC would also be an option. TAC does require growth factors but has about the same treatment duration as dose-dense therapy, and I would use this regimen.
We also saw in San Antonio that FEC-docetaxel was significantly better than the standard six cycles of FEC. So this is also a very legitimate treatment option. In the higher risk patient, I would pick one of these regimens, and I tend to use AC 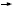 docetaxel. docetaxel.
— Stephen E Jones, MD
The simplest adjuvant chemotherapy regimen is AC or AC followed by paclitaxel. I’m a believer that dose-dense scheduling does have a role to play in terms of improving outcome. It’s less toxic in terms of myelosuppression. It is more expensive, but that’s the route I go. I haven’t been using TAC. Nobody has any good evidence that TAC is better than AC followed by paclitaxel or AC followed by dose-dense paclitaxel.
— Charles L Loprinzi, MD
Switching from tamoxifen to aromatase inhibitors
Generally I believe postmenopausal patients with ER-positive disease on tamoxifen for several years would benefit from switching to an aromatase inhibitor. You have a choice of exemestane or anastrozole. It’s difficult from the data at the present time to do a direct comparison between the two studies, but we can say that they’re both effective.
After five years of tamoxifen, our view is that any patient, other than somebody with a Grade I node-negative breast cancer, would benefit from extended adjuvant treatment with letrozole. If a patient with high-risk disease has been off tamoxifen for a year or two, I would still consider switching to “delayed adjuvant” therapy with letrozole.
— J Michael Dixon, MD
In selecting an aromatase inhibitor, I consider the clinical setting and generally choose the agent that was studied in that setting. That was simple when we had data from just the ATAC, IES and MA17 trials. I would select anastrozole for up-front therapy, exemestane after two or three years and letrozole after five years of tamoxifen.
Of course, that was just an artifact of the way the studies were designed and reported, and now we have other trials with data on different products in different settings, including the BIG-01-98 and ARNO trials. The data from these trials are incredibly similar to the data from the initial studies, so it may be a class effect and it may not matter which agent we choose.
— Harold J Burstein MD, PhD
At the two-year point, switching either to exemestane or anastrozole is an option, but I prefer exemestane, because the exemestane study is the most mature with the longest follow-up and a near survival difference.
For a patient who has received five years of tamoxifen, letrozole is the only drug studied, and I use it in women with node-positive and high-risk, node-negative disease, but I think the MA17 data has become a little fuzzier at this point in time because no survival difference was seen in women with node-negative disease.
With seven studies of adjuvant aromatase inhibitors, I believe oncologists will start mixing and matching, thinking there is a class effect and that these agents are all the same. I’m not sure that’s necessarily true, and I still try to follow the data. However, if someone has intolerance to one of the aromatase inhibitors, I feel pretty comfortable switching them to another that they can tolerate.
— Stephen E Jones, MD
Click here to see image
Based on the recent letrozole and exemestane data, the issue arises whether we should switch women to an aromatase inhibitor after either two to three years or four to five years of tamoxifen. At this point, the survival data is somewhat limited, but the disease-free survival data certainly favors that type of approach. The unknown, of course, is that neither of those large randomized trials had a control arm with an aromatase inhibitor alone. Therefore, I believe the question of whether tamoxifen adds anything to an aromatase inhibitor remains outstanding.
Nonetheless, if a woman who has started on tamoxifen gets into trouble, I don’t hesitate to switch her over to an aromatase inhibitor unless there is an absolute contraindication. Even when women do fine on tamoxifen, after the second or third year, I will have a discussion with them to see whether they have an interest in switching over. I don’t force that approach on them because these patients certainly could finish up five years of tamoxifen, and by that time, we can anticipate having further discussion.
— Gary Lyman, MD, MPH
The data presented at ASCO and San Antonio support the concept that switching to an aromatase inhibitor rather than continuing tamoxifen is beneficial. I switch to either exemastane or anastrozole. I don’t see any difference between the two when looking at the data. I would also consider switching to an aromatase inhibitor at one or four years of tamoxifen.
In terms of what to do at five years, I don’t have a clear rule as to recommending post-tamoxifen letrozole.
— Generosa Grana, MD
If a patient has completed five years of adjuvant tamoxifen, I pull out the calculator and inform the patient what to expect from their breast cancer in the next 10 years, with and without letrozole. Then I discuss the pros and cons of therapy, and it’s pretty easy to come to a decision.
I suspect most of these patients in my practice will opt for continued therapy, but it’s somewhat age-dependent. We have some bright, “salt-of-the-earth” type women here in the Midwest and they may be 78 years old and say, “Look, nobody’s going to live forever — and I don’t have a lot of funds, so let’s not do it.” I feel quite comfortable with that decision if there are a lot of competing causes of mortality, and it doesn’t mean you can’t always start an aromatase inhibitor if the patient develops recurrent disease.
— Charles L Loprinzi, MD
ER testing
We generally count any staining as ER-positive, but our pathologists will note if it is a low positive. You worry whenever you see low positives that the patient is not going to receive the same benefit as someone who has a high positive, even though it’s not very well data-driven. I will offer hormonal therapies to patients with low positive tumors, but will feel less comfortable with that as a mainstay, depending on the risk. Certainly, I’d be more inclined to add chemotherapy to hormonal therapy for a patient with node-positive disease or even one with node-negative disease if she had a big enough tumor or other high-risk features.
— Ann Partridge, MD
I rely on my pathology department to determine ER positivity, and I am willing to accept any ER positivity as positive, although I’m much more concerned with an ER positivity at one to five percent. At those levels I’m more likely to favor chemotherapy in addition to hormonal therapy.
— Generosa Grana, MD
Our institution pretty much still is basing our assessment on IHC assays. I don’t have a firm threshold for treatment, but certainly anything under 10 percent is negative as far as I’m concerned.
Even for a patient with 10 to 30 percent cells staining positive, I will tell them, “You’re technically positive, but I don’t think I would rely on hormonal therapy alone, if there’s any level of risk for the future.”
There has been data, some of which was presented at San Antonio, clearly suggesting that perhaps we should take another look at quantitative ER and PR, particularly with regard to the relative value of the aromatase inhibitors compared to a SERM. We’re not at that point at our institution, but I think if the data matures and suggests a benefit from quantitative measurements, we’ll probably go back to either doing that or reporting both and discussing them with the patient.
— Gary Lyman, MD, MPH
Most pathology laboratories have their own criteria for defining ER positivity and, to varying degrees, may differentiate percentages. Most of them are using immunohistochemical stains nowadays, and, one to 10 percent is considered weakly positive. Greater than 10 percent staining at our institution is considered positive.
There’s more and more information coming out to suggest that the degree of positivity may be important. This information seems like it has gone back and forth over the last 10 years. But effort has gone on to see if we can more routinely get different classifications of positivity, from 10 percent on up to near 100 percent.
There’s never an absolute in this business. Administering treatments such as hormonal therapy depend on a number of factors, including clinical presentation, but over 10 percent of cells staining is positive. If I have a patient with metastatic disease that’s lung-only, the ER is weakly positive and there’s no burning need for chemotherapy, it may well be reasonable to use hormone therapy. So I believe there are degrees of comfort in decision-making.
— Charles L Loprinzi, MD
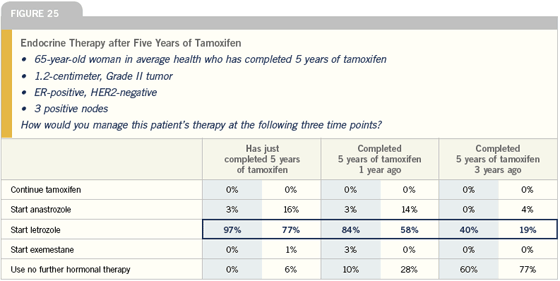
Bone density monitoring and the aromatase inhibitors
I will generally steer a woman towards tamoxifen who comes to me with well-advanced bone demineralization, osteopenia or frank osteoporosis. However, for a woman without major problems, I will order a baseline bone density evaluation either before or within the first several months of starting her on an aromatase inhibitor. I then repeat those studies on an annual basis.
I do not use bisphosphonates for every woman I start on an aromatase inhibitor. The data clearly shows that most women don’t need them and using them will add to the cost, morbidity, number of the visits and need for additional medication.
If clinically relevant changes begin to occur, I will discuss with the patient whether or not to add a bisphosphonate to try to stabilize or reverse some of the effects, or to make a change in the hormonal regimen.
— Gary Lyman, MD, MPH
Well before I used adjuvant aromatase inhibitors, I have taken it as my responsibility to monitor bone density. I have a much younger population of breast cancer patients who have been put through early menopause with chemotherapy. So ever since I started practice, it was my routine to make that part of my checklist with each follow-up.
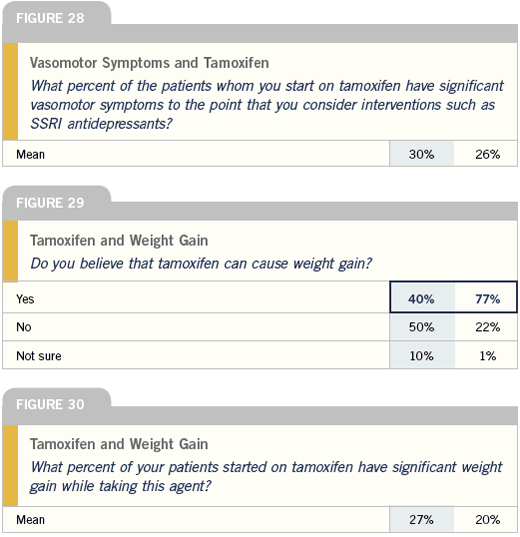
I truly haven’t changed my practice with the use of AI’s. All women who are postmenopausal at the end of their primary treatment receive a DEXA scan. Most commonly, I repeat the scan every two years, but in patients with low bone density, I’ll do it after a year or so. I’m not prophylactically starting patients on bisphosphonates if they’re on an aromatase inhibitor, because not all women need it. I’m starting it if the bone density drops.
— Julie Gralow, MD
One of the major side effects of aromatase inhibitors is accelerated osteoporosis and risk of osteoporotic fracture; however, two important points need to be made. First, the risk of osteoporotic fractures is approximately 0.5 to one fracture per hundred women per year, which is roughly the difference in disease-free survival that most of the studies also show.
Secondly, we don’t know what the impact of these drugs will be on the bones 15 years from now. We have limited data for three to five years of follow-up in all these trials, and, for the most part, it’s reasonably reassuring. However, remember that women who go through menopause in their early fifties lose a lot of bone rapidly, but they don’t develop osteoporosis or osteoporotic fractures until their seventies. We have no data on what will happen 10 years after a 57-year-old woman begins taking an aromatase inhibitor, and we have to be respectful of that lack of data.
When I treat a women with aromatase inhibitors, I generally order a baseline bone mineral density study within the first two months of initiating therapy and then repeat it a year or two later. As for treatment, I follow the standard WHO guidelines as to when to begin therapy for osteoporosis, and osteopenia, and I generally use an oral bisphosphonate.
— Harold J Burstein MD, PhD
Tolerability and adherence with tamoxifen
Based on clinical trial data and my own experience, I believe there are fewer side effects with the aromatase inhibitors than tamoxifen. Certainly there is less concern about uterine and thromboembolic complications, and I have found that there are usually fewer complaints about hot flashes or the severity of the hot flashes from women on anastrozole. Approximately 10 percent of women will have enough difficulty from their hot flashes or other symptoms that they will not want to continue tamoxifen; however, the majority of women still do very well on it.
— Gary Lyman, MD, MPH
Adherence is such a complex thing, and it’s going to become increasingly important in oncology because of the greater use of oral drugs. I did a study using an insurance and pharmacy database from New Jersey and Pennsylvania, looking at adherence with tamoxifen. The database predominantly contained patients who were on Medicare or Medicaid, so the old and the poor.
Adherence was actually better for women who were taking tamoxifen in their first year than most other chronic therapies. For most chronic therapies for conditions such as heart disease and hypertension, people take approximately 50 percent of their drug.
In our study, we found that overall adherence was a little over 80 percent, but that a substantial proportion, nearly 25 percent of our patients, took less than 80 percent per year. Some of this had to do with side effects, but the literature on adherence in oncology is kind of all over the place. Even with severe side effects, some people adhere while with minimal side effects others don’t.
— Ann Partridge, MD
Systemic therapy for DCIS
We do not assess hormone receptor status for every patient with DCIS, but this is an evolving area. Generally, when a woman with a high-grade DCIS is referred to me, I do try to obtain receptor status, and, if the receptors are positive, I will initiate a discussion about the potential benefit of tamoxifen in terms of delaying or preventing local recurrence with hormonal therapy.
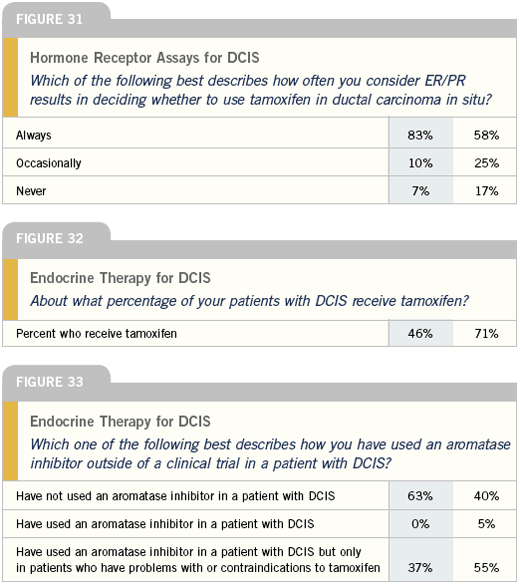
Certainly, the ATAC trial and other data clearly shows that SERMs are not the only hormonal agents that reduce the risk of contralateral disease or second malignancies. Aromatase inhibitors do that and probably do it a bit better than tamoxifen. So if a woman with a high-grade DCIS is concerned about a local-regional recurrence or needing a full mastectomy, I will discuss the data and the possibility of starting adjuvant hormonal therapy. Outside of that type of situation, I don’t use it routinely unless a woman is interested in chemoprevention.
— Gary Lyman, MD, MPH
DCIS is so stressful for patients, and these women have high levels of anxiety. In terms of systemic treatment, the only option available at this time is tamoxifen, based on the best data available from NSABP-B-24. I generally go through that with patients. If a person’s had a mastectomy, then all they’re doing is preventing a recurrence in their contralateral breast with tamoxifen. There really is no data that tamoxifen prevents systemic recurrences, because they are so rare. It probably does in a few people, but that’s not the main reason to be treated. I usually talk to patients about it as a more local issue, because for the vast majority of women, their greatest risk is their local risk. So if they’ve had a mastectomy, I say, “Sure, you can take tamoxifen for that two-percent benefit in terms of prevention, but it also carries risks with it.”
I check hormone receptor status and would not discuss tamoxifen with someone with hormone receptor-negative DCIS. The data reveals that it’s not helpful, based on the subset from B-24. I do talk to a lot of women about tamoxifen, and I’d say about 50 to 60 percent of the patients I offer it to decide to take it.
Currently, the aromatase inhibitors are being studied for DCIS, but no data is available yet. Do I think it’s probably going to show a benefit? Sure, but I wouldn’t use them until the studies report a benefit.
— Ann Partridge, MD
For the DCIS population and those who come in with ductal hyperplasia with atypia, I do have a discussion about chemoprevention and will try to present all the issues, both pro and con. Since there is limited data at this point in terms of the aromatase inhibitors for chemoprevention, unless a woman has a contraindication or intolerance to a SERM, I would not use an aromatase inhibitor at this point. Do I think it’s going to prove effective in that role? Probably. But for a woman who hasn’t been treated for an invasive cancer, I’m much less willing to gamble with her bones than I would be for a woman who’s at risk for recurrence of a true invasive breast cancer.
— Gary Lyman, MD, MPH
I use tamoxifen for patients who want endocrine therapy for DCIS. Based on data from the adjuvant setting, the NSABP is conducting a large trial to determine if there is a role for aromatase inhibitors in the treatment of DCIS, which I believe is a very reasonable study. The good news for patients with DCIS is that most women do very well. With the current surgical and radiation therapy techniques, the risk of recurrence is only five to 10 percent, and we can more or less cut that in half with tamoxifen. The challenge is, “Can we do much better than that?”
— Harold J Burstein MD, PhD
Select publications
|

